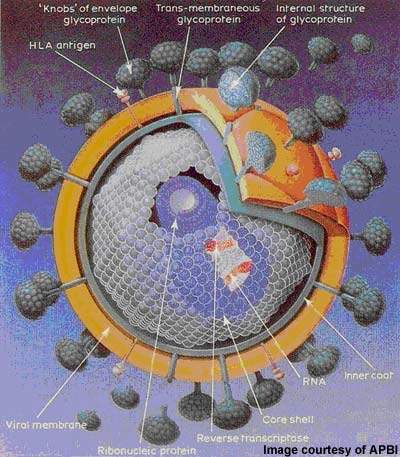
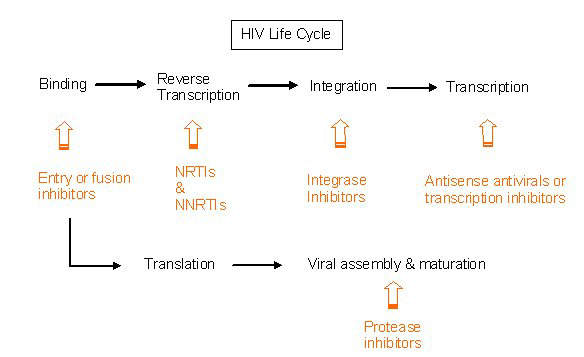
Under development by Incyte Corporation, INCB9471 is an experimental small molecule drug indicated for the treatment of infection due to the human immunodeficiency virus (HIV). It is a member of a new class of oral HIV drugs called CCR5 receptor antagonists or inhibitors.
INCB9471 is currently in phase II development, where it has shown promising evidence of antiviral efficacy.
CCR5 INHIBITORS REPRESENT A NEW CLASS OF HIV DRUGS
CCR5 receptor antagonists, of which Pfizer’s maraviroc was the first to be approved for clinical use, are unlike existing drugs for HIV. While other oral HIV drugs target the virus inside white cells, CCR5 inhibitors are designed to prevent the virus from entering uninfected CD4-T cells – the target for HIV.
The retrovirus gains entry to CD4-T cells via one of two obligate receptors: CCR5 and CXCR4. CCR5 receptor antagonists work by blocking the entry of HIV that binds exclusively to the CCR5 co-receptor (so-called R5-tropic HIV).
Scientists believe CCR5 antagonists may be of most value early in the course of HIV therapy, where R5-tropic HIV dominates. For example, more than 85% of treatment-naïve HIV patients have only R5 tropic virus detectable in their blood.
INCB9471 SHOWS SUSTAINED ANTIVIRAL EFFECTS
Results from the first of a series of phase II trials of INCB9471 in HIV patients suggest it possesses potent and prolonged antiviral effects against R5-tropic HIV in both treatment-naïve and treatment-experienced patients.
In this 14-day, 23-patient placebo-controlled study, administration of a 200mg once-daily dose of INCB9471 achieved rapid and prolonged reduction in viral load with a mean maximal decline of 1.81 log10 at day 16. Consistent with its long plasma half-life, viral load suppression continued well beyond the 14-day dosing period with a mean 0.81 log10 decline in viral load observed at day 28.
INCB9471 appeared safe and well tolerated in this small study. Previous phase I safety studies in volunteers had shown that single doses of up to 300mg and multiple doses of up to 200mg for ten days were well tolerated; no dose-limiting toxicity was observed.
MEETING THE CHALLENGE OF DRUG RESISTANCE
The advent of anti-retroviral drugs in the 1990s had a major impact on the treatment of HIV. While unable to eliminate the virus from the body, these drugs were nonetheless able to reduce symptoms, improve immune status and delay the onset of AIDS.
Although there are now a large number of approved drugs for treatment of HIV, the development of drug resistance can severely limit their clinical effectiveness.
Incyte’s INCB9471 has been found to effectively inhibit infection of cells by strains of R5-tropic HIV that have developed resistance to the four major classes of anti-retroviral drugs. These include protease inhibitors, nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs) and fusion inhibitors. Therefore, it potentially may be of benefit in HIV patients who have become refractory to standard HIV drug regimens.
MARKETING COMMENTARY
Since its discovery in the early 1980s, infection with HIV has reached epidemic proportions, particularly in developing countries. Estimates suggest that 42 million people are living with HIV/AIDS, of who around 3 million or more die each year. For HIV patients with access to treatment, the range of available therapies continues to grow. In the US, the market for HIV drugs is expected to more than double by 2011.
CCR5 receptor antagonists represent an exciting new class of oral drugs for HIV with an entirely different mode of action to existing HIV therapies. Although still at an early stage of clinical development, Incyte hopes that INCB9471 and its other CCR5 antagonists currently in development will join Pfizer’s maraviroc as a marketed drug.