AbbVie has announced that the first patient has been dosed in the Phase III Step-Up HS study evaluating upadacitinib in adults and adolescents with hidradenitis suppurativa (HS).
The Phase III trial (NCT05889182) will assess the change in disease activity of oral upadacitinib in patients with moderate to severe HS who have failed anti-TNF therapy.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The study will involve both adults and adolescents and AbbVie hopes to recruit 1328 patients across 275 sites worldwide. It is a randomised, placebo-controlled, double-blind study due to end in August 2027.
The study consists of three periods. In period one, the safety and efficacy of upadacitinib 30mg will be evaluated versus placebo. In period two, based on clinical response, patients will be put into three arms to receive upadacitinib 30mg, upadacitinib 15mg or a placebo following re-randomisation. Period three will be a long-term extension. The primary endpoint is at least a 50% reduction in the total abscess and inflammatory nodule (AN) count, no increase in abscess count and no increase in draining fistula count.
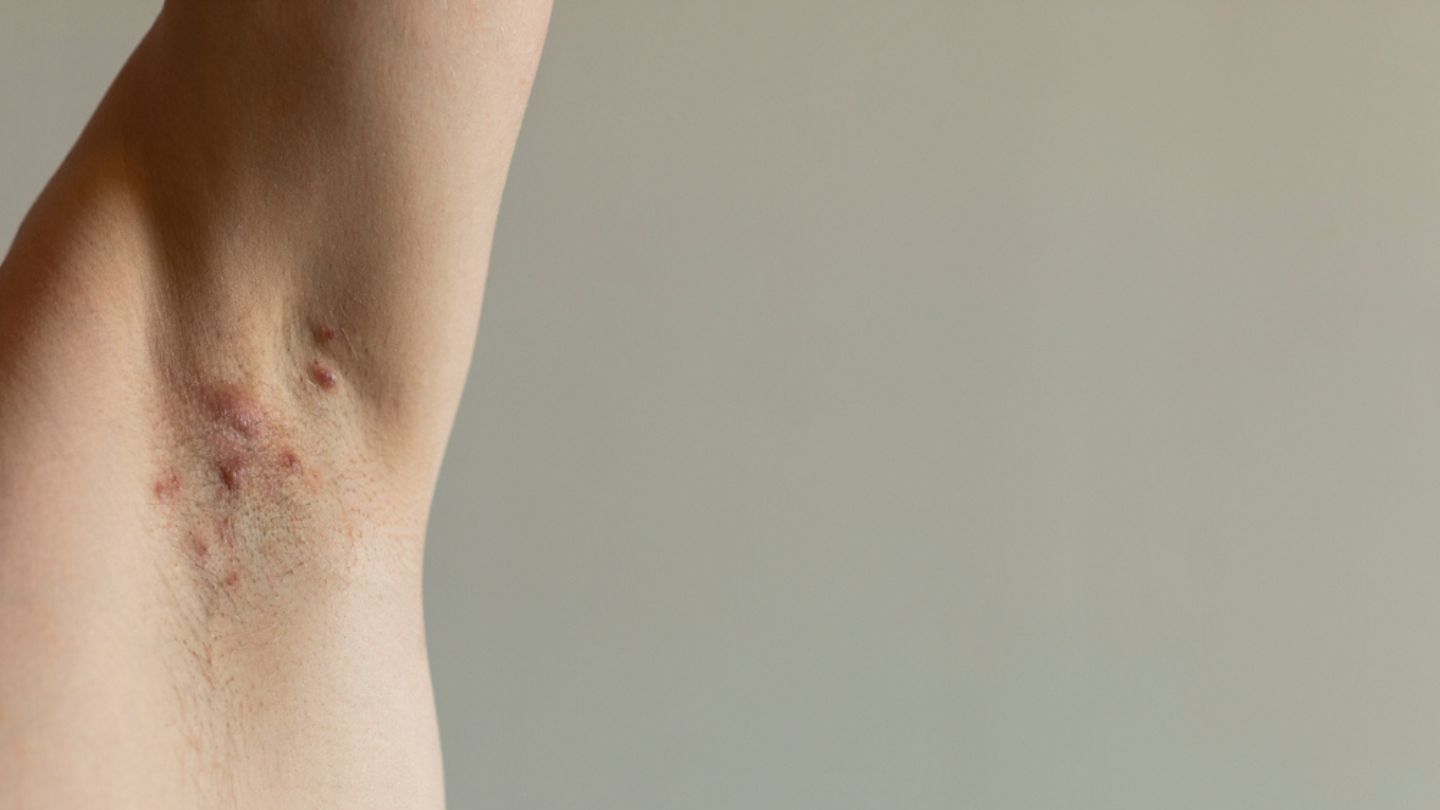
What is HS?
HS can appear as bumps, nodules or abscesses on the skin that leak fluid. They can lead to scarring and even connect under the skin by tunnels. They tend to develop where hair grows or skin rubs against skin.
AbbVie development and regulatory affairs senior vice-president and chief medical officer Dr Roopal Thakkar said: “Hidradenitis suppurativa is a chronic, inflammatory disease that often leads to irreversible skin damage and extreme pain for patients.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Leveraging our proven expertise in immunology and experience in HS, we continue to drive innovation and pursue the advancement of care in patient populations with difficult-to-treat immune-mediated diseases that have limited therapeutic options.”
Market details of upadacitinib
Known by the market name RINVOQ, upadacitinib is a selective Janus kinase (JAK) inhibitor. JAKs are intracellular enzymes that transmit signals arising from cytokine or growth factor-receptor interactions on the cellular membrane to influence cellular processes of haematopoiesis and immune cell function.
AbbVie is also conducting Phase III trials of RINVOQ in rheumatoid arthritis, atopic dermatitis, psoriatic arthritis, axial spondyloarthritis, Crohn’s disease, ulcerative colitis, giant cell arteritis, Takayasu arteritis, and systemic juvenile idiopathic arthritis. The use of upadacitinib in HS is not yet approved and its safety and efficacy have not been evaluated by regulatory authorities.





