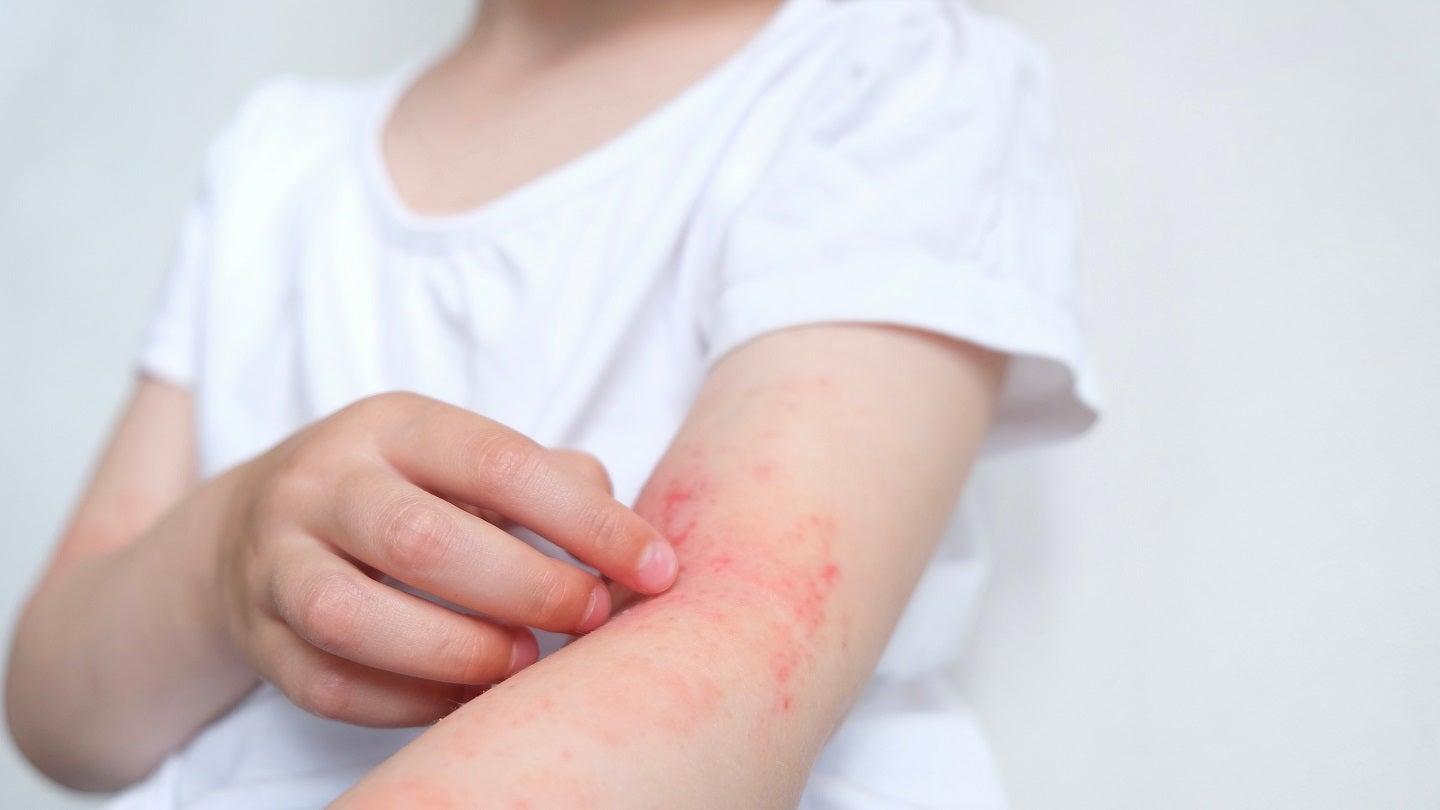
Aclaris Therapeutics has concluded the enrolment of 250 patients in the Phase IIb ATI-1777-AD-202 study of ATI-1777 to treat mild to severe atopic dermatitis (AD).
Aclaris is an investigational topical Janus kinase (JAK) inhibitor that is delivered as a spray-on solution to minimise systemic exposure.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The initiation of the vehicle-controlled Phase IIb trial was based on the results from the four-week Phase IIa trial of the topical soft JAK 1/3 inhibitor ATI-1777 in patients with moderate to severe AD.
Improvement in the modified Eczema Area and Severity Index (EASI) and minimal measurable systemic exposure were observed in the Phase IIa trial patients who received a 2% formulation of ATI-1777 twice daily.
In the Phase IIb study, Aclaris intends to further explore ATI-1777 in concentrations including 0.5%, 1% and 2%, as well as a once-daily regimen using the 2% formulation.
Being conducted across 34 US clinical trial sites, the trial enrolled adults and children from aged 12 years with mild, moderate or severe AD.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe percent change in EASI over a period of four weeks is the primary efficacy endpoint while the safety, efficacy and pharmacokinetics are the secondary measures of the study.
Aclaris chief medical officer Gail Cawkwell said: “We are very pleased to have completed the enrolment stage of our Phase IIb trial of ATI-1777 in atopic dermatitis, one of our two lead clinical development assets.
“With this achievement, we are positioned for a very exciting year end for our two lead assets, first with the data read-out of our Phase IIb trial of zunsemetinib (ATI-450) in rheumatoid arthritis expected in November, followed by the top-line results of the ATI-1777 trial expected around year end.”
The top-line results, which will include efficacy, safety and other preliminary data of ATI-1777, are expected to be released at the end of the year.
Aclaris plans to develop ATI-1777 as an emollient-containing spray formulation.





