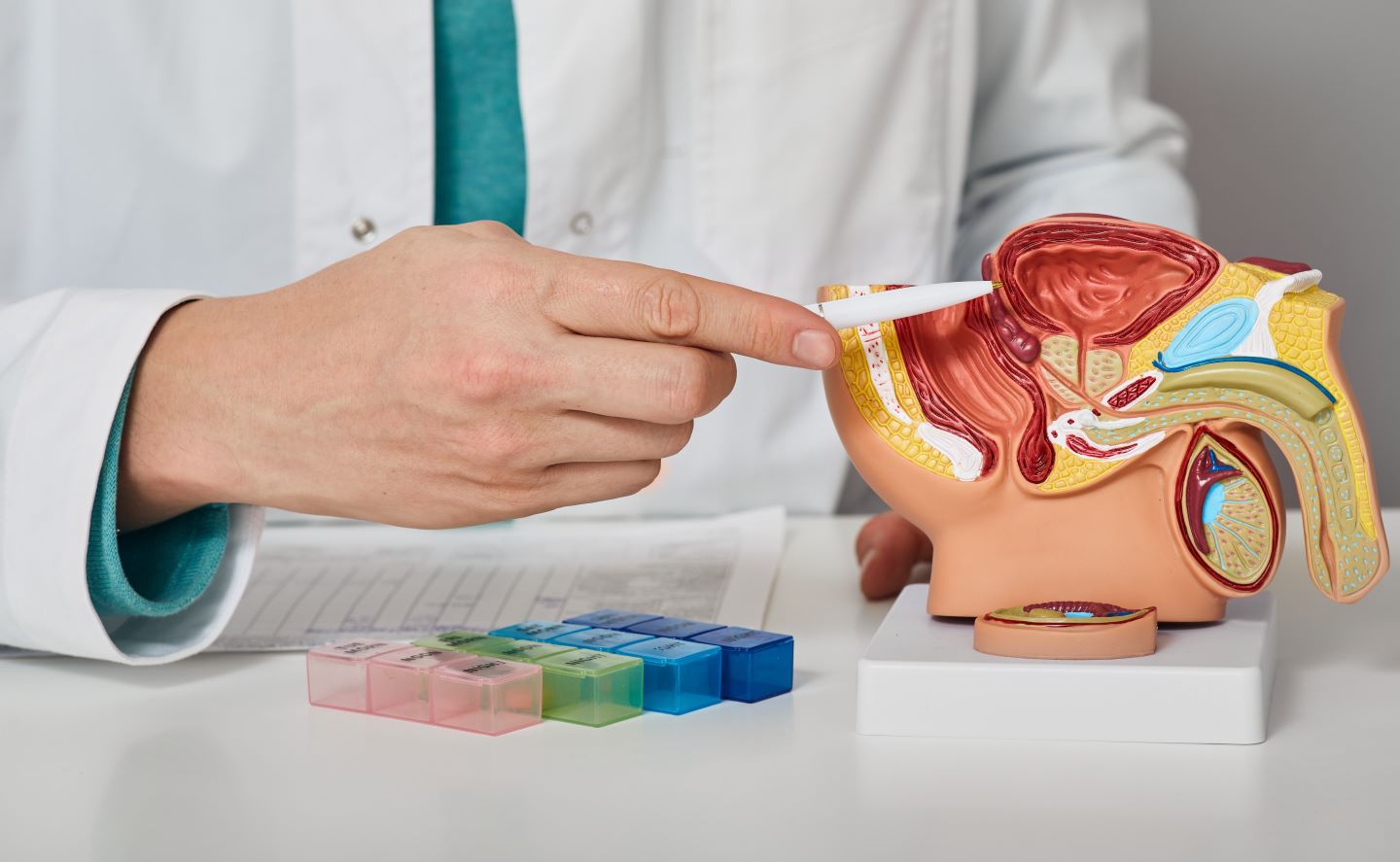
AdvanCell has dosed the first subject in its Phase I/II TheraPb clinical trial of ²¹²Pb-ADVC001 to treat PSMA-positive metastatic castration-resistant prostate cancer (mCRPC).
The open-label, multicentre trial will analyse the tolerability and safety and detect a recommended Phase II dose of the drug candidate as primary objectives.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Dosimetry measures, radiographic progression-free survival (rPFS), and prostatic-specific antigen (PSA) response will comprise the secondary objectives of the trial.
The company launched various study centres for the trial, which are now open for subject enrolment.
A targeted alpha therapy, ²¹²Pb-ADVC001 attaches to PSMA expressed in prostate cancer.
AdvanCell CEO Andrew Adamovich said: “The first patient dosed with an AdvanCell therapeutic candidate represents a major milestone for the company, our scientists, clinical trial sites, and our patients.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“We believe that ²¹²Pb is the ideal radioisotope to realise the full therapeutic power of targeted alpha therapy.
“This promise is strongly supported by preclinical results for ²¹²Pb-ADVC001, which demonstrate the potential for best-in-class safety and efficacy.”
Since 2020, the company developed the drug and entered a partnership with a radiopharmaceutical company on a supply chain for ²¹²Pb radioisotope and radioligand as well as a network for drug production.
AdvanCell also secured funding worth $13m from investors including Morningside and acquired a 35,000ft² manufacturing site.





