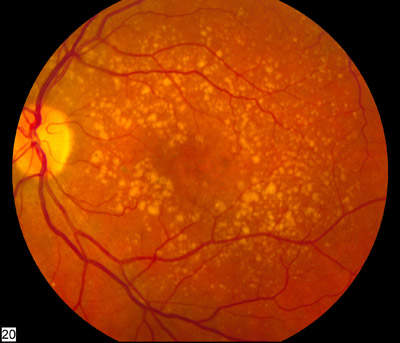
British artificial intelligence company BenevolentAI has announced its partnership with charity group Action Against Age-related macular degeneration (AAA) to develop treatments for age-related macular degeneration (AMD), the leading cause of blindness in adults in the UK.
Four vision loss charities, Blind Veterans UK, Fight for Sight, the Macular Society and Scottish War Blinded, formed AAA to increase funding for biomedical research into the treatment of AMD and its degenerative effects.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
“AMD is a complex and multi-factoral disease in which understanding of the pathogenesis remains unclear,” AAA development chair Rob Hunter told Drug Development Technology.
“Developing a new treatment is made more difficult as the unique anatomy of the eye creates challenges in both delivery and clearance of products. When combined with the progressive and irreversible degeneration of the macula, it creates a very difficult environment in which to develop a treatment.
“We’re confident that the assistance of BenevolentAI will rapidly advance our research into AMD and will help us identify new areas to focus research on.”
BenevolentAI’s bioscience arm, BenevolentBio, will use its AI technology platform to support AAA’s research, processing and reviewing vast amounts of information on the various trials, papers and additional data surrounding the research into AMD and its potential treatments.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Using our AI platform we are able to search vast online libraries of unstructured and seemingly unrelated data to create unique avenues of exploration for AMD research,” BenevolentBio CEO Jackie Hunter told Drug Development Technology.
“The technology is able to not only identify existing compounds of potential interest from drug libraries, but also generate completely novel treatments for disease too.”
Current treatment options for the disease are severely limited, and the rising number of sufferers makes the development of new therapies a growing need. It is predicted that by 2020 almost 700,000 people will have late-stage AMD, which is classed as when vision loss interferes with a patient’s ability to read, drive, recognise faces or perform many everyday tasks.
AMD is the third biggest cause of sight loss worldwide and the main cause of blindness in developed countries. More than 600,000 people have late stage AMD in the UK, with predictions for this figure to double by 2050 as life expectancy increases.





