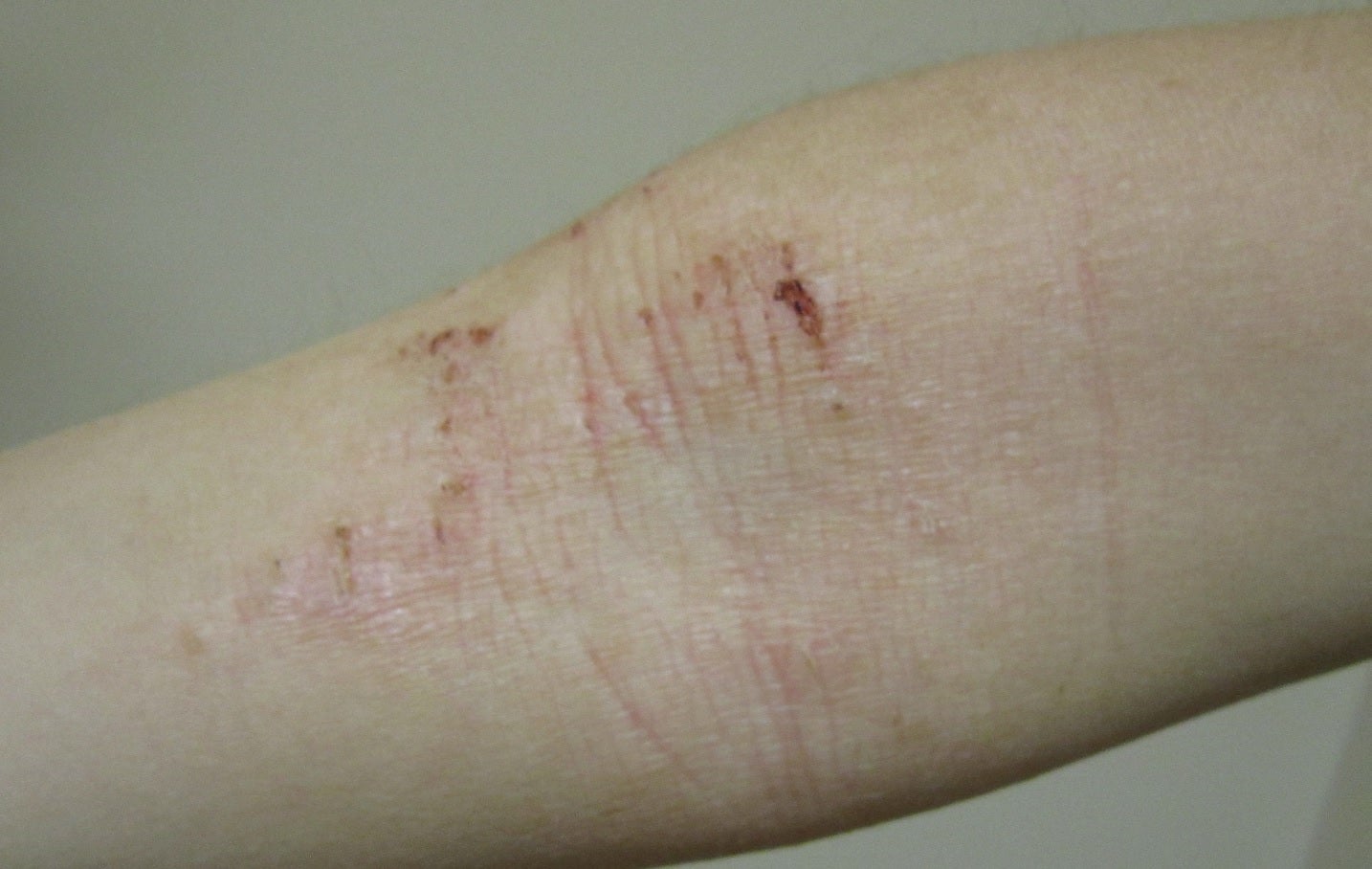
Aldeyra Therapeutics has enrolled the first participant in the Phase II clinical trial of investigational new drug, ADX‑629, to treat atopic dermatitis.
The two-part, adaptive, multicentre Phase II trial has been designed for assessing the efficacy and safety of ADX‑629 alone and along with standard of care in mild, moderate, or severe atopic dermatitis adult patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Nearly 10 participants will receive open-label ADX‑629 twice-a-day for 90 days in Part 1 of the trial.
Improvement in Investigator Global Assessment and Eczema Area and Severity Index scores will be some of the trial’s outcomes.
In Part 2, participants will be randomised to receive either ADX‑629 or placebo treatment twice-a-day for 90 days.
The company expects to receive top-line results from Part 1 in the second half of this year.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAldeyra Therapeutics CEO and president Todd Brady said: “Atopic dermatitis, a chronic hypersensitivity condition characterised by dry, itchy, and inflamed skin, affects an estimated 16.5 million adults and more than 9.6 million children in the United States.
“ADX‑629, if approved, would be the first RASP modulator and one of the few orally administered therapies indicated for the treatment of atopic dermatitis.”
The company stated that ADX‑629 is also currently being assessed in Phase II clinical trials to treat Sjögren-Larsson Syndrome, chronic cough, and idiopathic nephrotic syndrome.
It expects to obtain top-line results from the chronic cough trial in the first half of this year.
In the second half, a Phase II clinical trial of ADX-629 to treat moderate alcohol-associated hepatitis is anticipated to commence.





