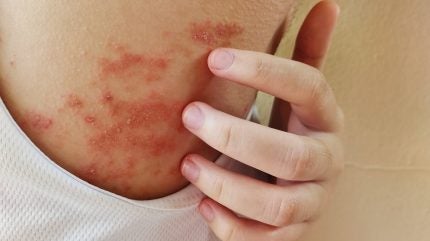

Amgen and Kyowa Kirin have announced initial top-line findings from the Phase III ASCEND trial assessing the investigational T-cell rebalancing therapy, rocatinlimab, aimed at treating adults and adolescents with moderate to severe atopic dermatitis (AD).
The ASCEND trial is part of the expansive ROCKET Phase III clinical programme, which includes eight trials.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
These studies are designed to determine the efficacy and safety of various dosing schedules of the therapy for patients with moderate to severe AD.
ASCEND involves approximately 2,600 subjects and focuses on assessing the long-term impact of rocatinlimab in 150mg and 300mg dosages, given to individuals at either four or eight-week intervals.
The preliminary analysis concentrated on the adult population who had completed 24 weeks of treatment in an earlier ROCKET trial and continued with ASCEND for an additional 32 weeks.
The primary goal of the trial was to assess the long-term safety of the therapy, with secondary endpoints assessed in adults who demonstrated a clinical response in the HORIZON or IGNITE studies and were subsequently re-randomised in ASCEND.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataA significant number of patients in this subgroup, who received ongoing standalone treatment of rocatinlimab, reported sustained benefits after one year of treatment. These benefits included improvements in itch, skin clearance, disease extent, and severity.
The companies are planning to present the comprehensive results at an upcoming medical congress or through a peer-reviewed scientific publication.
They said that rocatinlimab is also being investigated for other conditions, such as moderate to severe uncontrolled asthma and prurigo nodularis, where T-cell imbalance is believed to be a fundamental cause of inflammation.
The initial antibody was discovered through a joint effort between Kyowa Kirin and the La Jolla Institute for Immunology in the US.
Amgen research and development executive vice-president Jay Bradner said: “Atopic dermatitis is a heterogeneous disease where many patients still lack adequate control with current therapies.
“These findings add to our understanding of the role OX40 inhibition can play in addressing the underlying drivers of this chronic disease and provide further information on rocatinlimab’s durability of response and long-term safety profile, which we will continue to monitor.”
In March 2025, Amgen and Kyowa reported that the 24-week Phase III IGNITE trial assessing two dose strengths of rocatinlimab in individuals with moderate to severe AD met the co-primary endpoints.
Cell & Gene therapy coverage on Clinical Trials Arena is supported by Cytiva.
Editorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.





