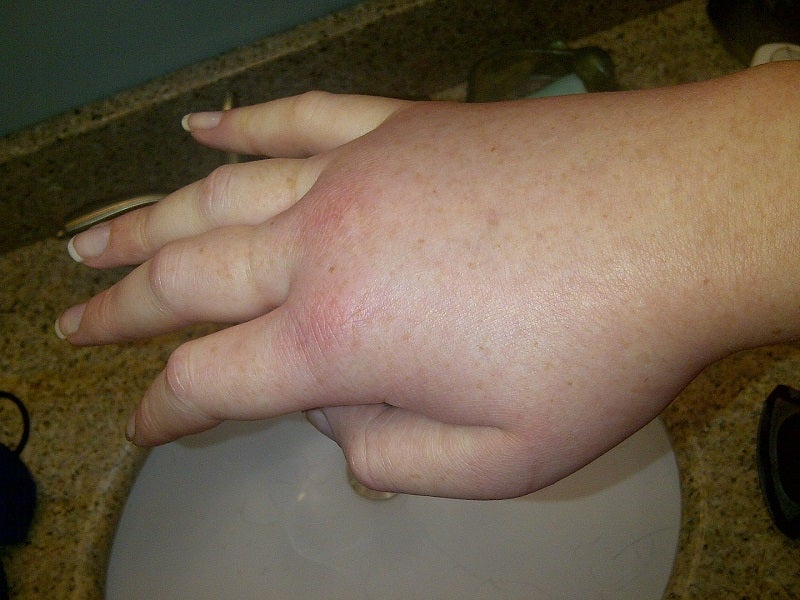
Astria Therapeutics has announced the start of the Phase Ib/II ALPHA-STAR clinical trial of STAR-0215 to treat hereditary angioedema (HAE) patients.
The open-label, global Phase Ib/II proof-of-concept trial has been designed for assessing STAR-0215 as a potential long-acting treatment to prevent HAE attacks.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It is enrolling HAE type I and II patients to assess the pharmacokinetics, tolerability, safety, assessments of quality-of-life, pharmacodynamics, and changes in HAE attack rate.
The company stated that the results from up to 18 subjects will assess the safety and efficacy and will be compared against the data collected during the run-in period.
It expects to receive initial data from the single and multiple dose cohorts of the ALPHA-STAR trial in middle of next year.
Astria Therapeutics chief medical officer Chris Morabito said: “Our vision for STAR-0215 is to develop a long-acting, safe, and effective preventative therapy that normalises the lives of people living with HAE.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“After seeing promising Phase Ia clinical results at the end of last year, we are proud to be taking the next step forward by evaluating STAR-0215 in HAE patients in our proof-of-concept clinical trial.
“We believe that STAR-0215 has the potential to change the way that people live with their HAE.”
STAR-0215 is a monoclonal antibody plasma kallikrein inhibitor that is being developed to treat HAE.
The company said that the preliminary data from a Phase Ia trial in healthy subjects supports the target profile of the antibody, including a best-in-class PK profile, a long-acting preventative therapy, and dosing once every three months or less.
Astria intends to assess the potential administration of STAR-0215 for six months in additional cohorts in the Phase Ia trial, based on data seen to-date.
The preliminary data from the trial is anticipated in the fourth quarter of this year.





