Attralus has dosed the first subject in the Phase I clinical trial of its lead pan-amyloid removal (PAR) therapy, AT-02, in healthy participants and systemic amyloidosis (SA) patients.
The two-part trial will assess the safety, tolerability and pharmacokinetics (PK) of single increasing doses of AT-02.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Part 1 of the trial is a double-blind, single-centre, single-ascending dose study enrolling healthy volunteers (HV). It will analyse the PAR therapeutic’s safety, tolerability and PK.
The open-label, single-ascending dose Part 2 study will enrol SA patients to evaluate the safety, tolerability and PK of AT-02 and detect the maximum tolerated dose (MTD).
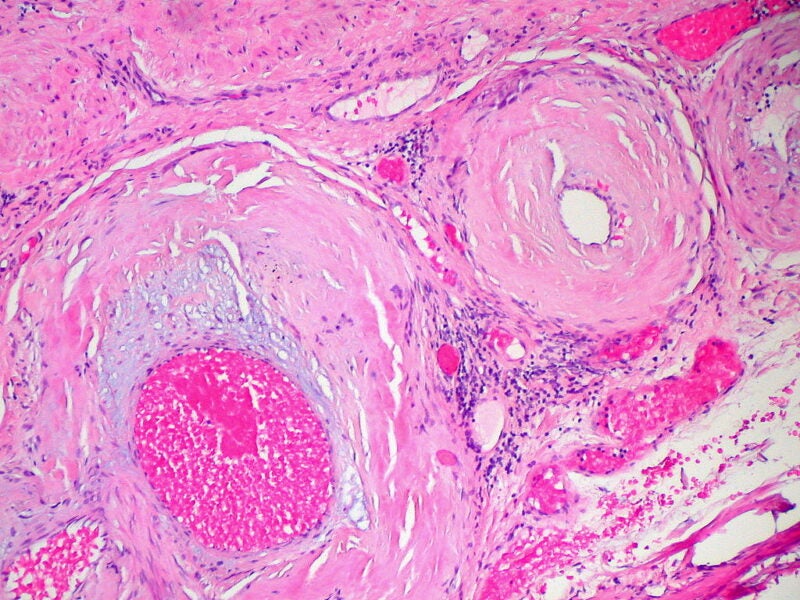
A humanised, full-length, recombinant immunoglobulin 1 (IgG1) monoclonal antibody (mAb) fusion protein, AT-02 is in the developmental stage for SA treatment.
It demonstrated to have subnanomolar binding potency to ATTR and AL amyloid as well as opsonise amyloid extracts boosting amyloid phagocytosis mediated by macrophage.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAccording to in vivo preclinical findings, the therapy offered substantial amyloid decline in mouse models of human AL amyloidoma and systemic AA amyloidosis.
Nearly 50% reduction in local cardiac amyloid was observed in mice with progressive systemic AA amyloidosis, which received AT-02.
The treatment also substantially decreased renal and hepatic amyloid and organ impairment versus untreated animals.
Attralus chief medical officer Gregory Bell said: “For systemic amyloidosis patients today, approved therapies target precursor protein production, reducing the formation of new amyloid, but there is a significant unmet need for new therapies that can remove the existing toxic amyloid fibrils, which cause organ damage and mortality in patients.
“AT-02 is designed to bind to all types of amyloid and induce immune-mediated phagocytosis.
“Based on the preclinical data, we believe AT-02 has the potential to remove existing amyloid and improve organ function and clinical outcomes for patients.”
Systemic amyloidosis comprises various groups of rare ailments that arise due to the build-up of toxic amyloid deposits in tissues and organs.



