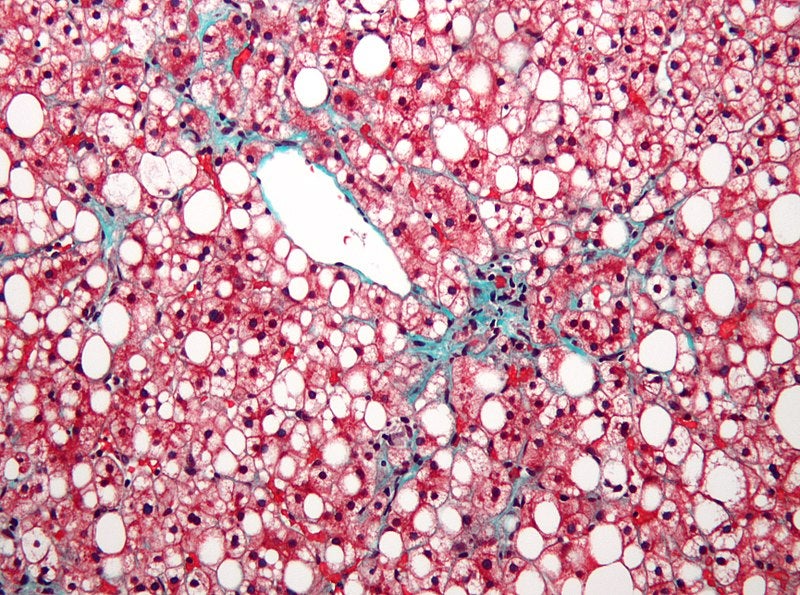
Biotechnology company Axcella has announced positive top-line data from the AXA1125-003 clinical study assessing the impact of AXA1125 and AXA1957 product candidates for the treatment of adult patients with non-alcoholic fatty liver disease (NAFLD).
For the placebo-controlled, randomised, multi-arm clinical study, a total of 102 NAFLD subjects with presumed nonalcoholic steatohepatitis (NASH), based on inclusion criteria, were enrolled.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The subjects were dosed in a 2:2:2:1 ratio and given AXA1125, one of two AXA1957 doses, or placebo twice daily for 16 weeks.
It assessed the impact of the distinct product candidates on safety, tolerability, and effects on structures and functions of the liver.
They are proprietary compositions of amino acids and derivatives designed to support liver health.
The study results showed that the candidates were generally well-tolerated, with sustained reductions noted for both of them versus placebo in key biomarkers of metabolism, inflammation, and fibrosis over 16 weeks.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAxcella chief medical officer Manu Chakravarthy said: “In AXA1125-003, we were seeking to evaluate safety and tolerability while also determining what differential responses may be seen from AXA1125 and AXA1957 across markers of metabolism, inflammation and fibrosis.
“It is gratifying that multi-factorial activity and a favourable tolerability profile were noted for AXA1125 in this multi-arm, placebo-controlled randomised study, which replicates findings from our previous clinical study in subjects with NAFLD and type 2 diabetes.
“We believe that these data, coupled with AXA1125’s oral route of administration and favourable tolerability profile to date, reinforce its potential to meaningfully improve the lives of patients with NASH.”
The company plans to engage with FDA regarding IND submission for AXA1125, a proposed Phase IIb clinical trial in adult nonalcoholic steatohepatitis (NASH), and a paediatric development programme.





