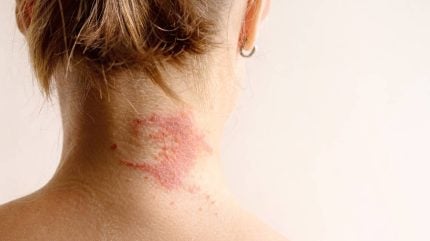
Bambusa Therapeutics has announced the dosing of its first healthy volunteers as part of a Phase I trial on its half-life extended bispecific antibody therapy for atopic dermatitis (AD).
The randomised, placebo-controlled trial (NCT06808477) is set to recruit approximately 98 volunteers, as well as adults living with AD, from a single site in Australia to test safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD) and preliminary clinical activity of BBT001, the company’s lead candidate. Data is expected in H2 2025.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The news follows just a week after the start-up secured $90m in a Series A financing round to propel its pipeline candidates including BBT002, another bispecific antibody designed as a “platform in a molecule” with intended applications across respiratory, dermatology, and gastroenterology diseases.
Shanshan Xu, CEO of Bambusa Therapeutics, said: “The early initiation of the BBT001 Phase I clinical trial is a significant milestone for Bambusa as we continue to execute with excellence.
“BBT001 represents a potential breakthrough in atopic dermatitis treatment by leveraging innovative antibody engineering to engage multiple, clinically validated, non-overlapping mechanisms of action. Our goal is to deliver faster onset of symptom relief, superior efficacy, and improved dosing convenience that could ultimately redefine the standard of care for AD patients.”
The single ascending dose (SAD) and multiple ascending dose (MAD) study follows pre-clinical studies in which the company claims BBT001 demonstrated its best-in-disease potential, offering enhanced efficacy and improved dosing convenience compared to currently approved therapies.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataResearch by the National Eczema Association estimates that approximately 10% of the US population has some form of eczema. Itchy or inflamed skin is the most notable symptom of the skin condition. In a study, 60.5% of adults with AD reported severe or unbearable itch in a two week period, 86% reported daily itch and 63% reported itching at least 12 hours per day.
GlobalData’s Pharmaceutical Intelligence Centre estimates that total global sales for AD treatments brought in more than $15.4bn by the end of 2024, with that figure estimated to rise to $38bn by the end of 2030. That market is largely dominated by Sanofi’s Dupixent (dupilumab), a monoclonal antibody, which in 2024 alone brought in revenue of $14.1bn.
GlobalData is the parent company of Clinical Trials Arena.
Beyond BBT001 and BBT002, the company is also working on BBT003 and BBT004. Both are being developed for inflammatory bowel disease and rheumatological conditions.
Elsewhere in the field of AD therapies, Nektar Therapeutics received fast track designation for Rezpeg (rezpegaldesleukin) in AD on 10 February. A Phase IIb trial of the therapy reached target enrolment in patients living with alopecia areata.





