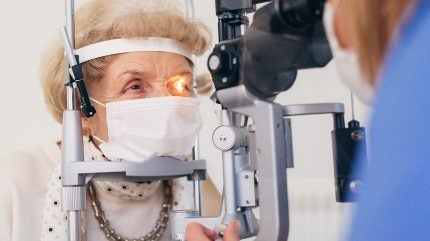
German pharmaceutical company Boehringer Ingelheim has started the JADE Phase II clinical trial to evaluate BI 1584862 as a first-in-class oral treatment for geographic atrophy (GA).
Taking place across 21 US locations, the study aims to determine the therapy’s safety and efficacy in preserving patients’ vision.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The randomised, double-masked trial is enrolling adult subjects aged 55 years and older who have been diagnosed with GA through retinal imaging.
Its objective is to identify the optimal dose of BI 1584862 and observe its impact on improving the eyes of GA patients.
Subjects will be divided into four groups at random, with an initial allocation to either a BI 1584862 group or a placebo group, followed by additional randomisation into two more BI 1584862 groups and another placebo group.
Over 12 months, subjects will make 13 visits to the trial site, where the progression of their eye disease will be monitored and any health issues will be recorded.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAt these visits, doctors will use fundus autofluorescence and optical coherence tomography imaging methods to capture detailed images of the retina.
They will measure retinal areas to track changes in healthy and atrophying regions, comparing these changes across the treatment groups.
Boehringer Ingelheim’s medicine and eye health global head Patrick Bussfeld said: “At Boehringer Ingelheim, we recognise the need for treatments that address the diverse needs of GA patients and the medical community.
“Our commitment is reflected in our Phase II clinical trials investigating two distinct compounds, BI 1584862 and BI 771716, each targeting different pathways.
“By exploring multiple avenues, we are seeking to offer more individualised treatment options and, ultimately, improve the quality of life for people living with GA.”
Boehringer Ingelheim’s drug candidate zongertinib recently showed a durable response of longer than 14 months when given to patients with advanced or metastatic solid tumours and non-small cell lung cancer.





