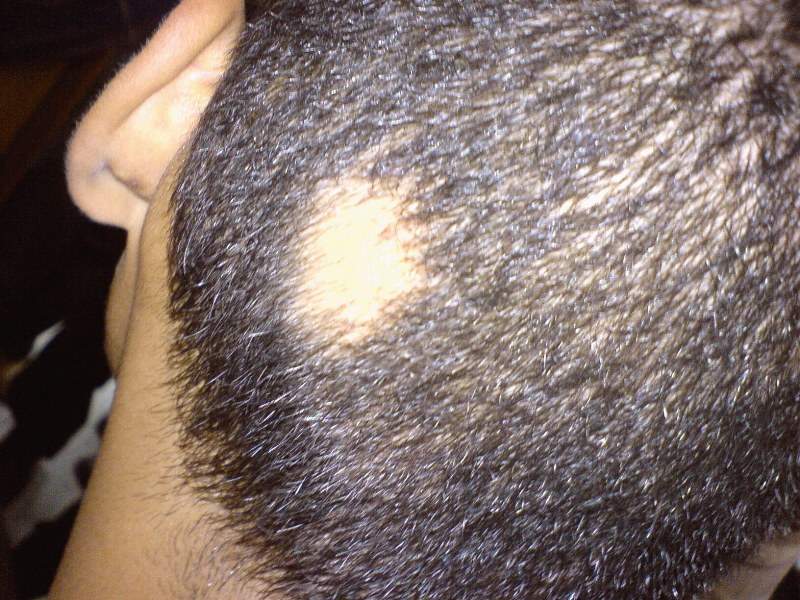
Concert Pharmaceuticals has reported positive interim topline results from the first two cohorts of its Phase IIa trial assessing CTP-543 in patients with moderate-to-severe alopecia areata.
The trial met its primary efficacy endpoint with 47% of patients treated with an 8mg twice-daily dose of CTP-543 achieving a ≥50% relative reduction in their overall severity of alopecia tool (SALT) score at 24 weeks compared to placebo.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
In the trial’s 4mg cohort, 21% of patients achieved a ≥50% relative reduction in their overall SALT score from baseline, though these results were not significantly different to placebo.
It was also found that the response observed in the 8mg twice-daily dose was significantly different from the 4mg twice daily dose.
The average baseline SALT score for all patients included in the trial was around 88.
Common side effects reported included headaches, upper respiratory tract infection, cough, acne and nausea. No serious adverse events were reported.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPatients are currently being dosed in the final 12mg twice-daily cohort.
Concert Pharmaceuticals chief development officer James Cassella said: “We believe that the extent of efficacy and tolerability of CTP-543 to date supports its further development.
“We look forward to completing the final dosing cohort in this Phase IIa trial and advancing the programme into later stage clinical trials.”
The Phase IIa trial features a double-blind, randomised, placebo-controlled, sequential-dose study design.
Complete results from the trial are expected to be available in the third quarter of next year.





