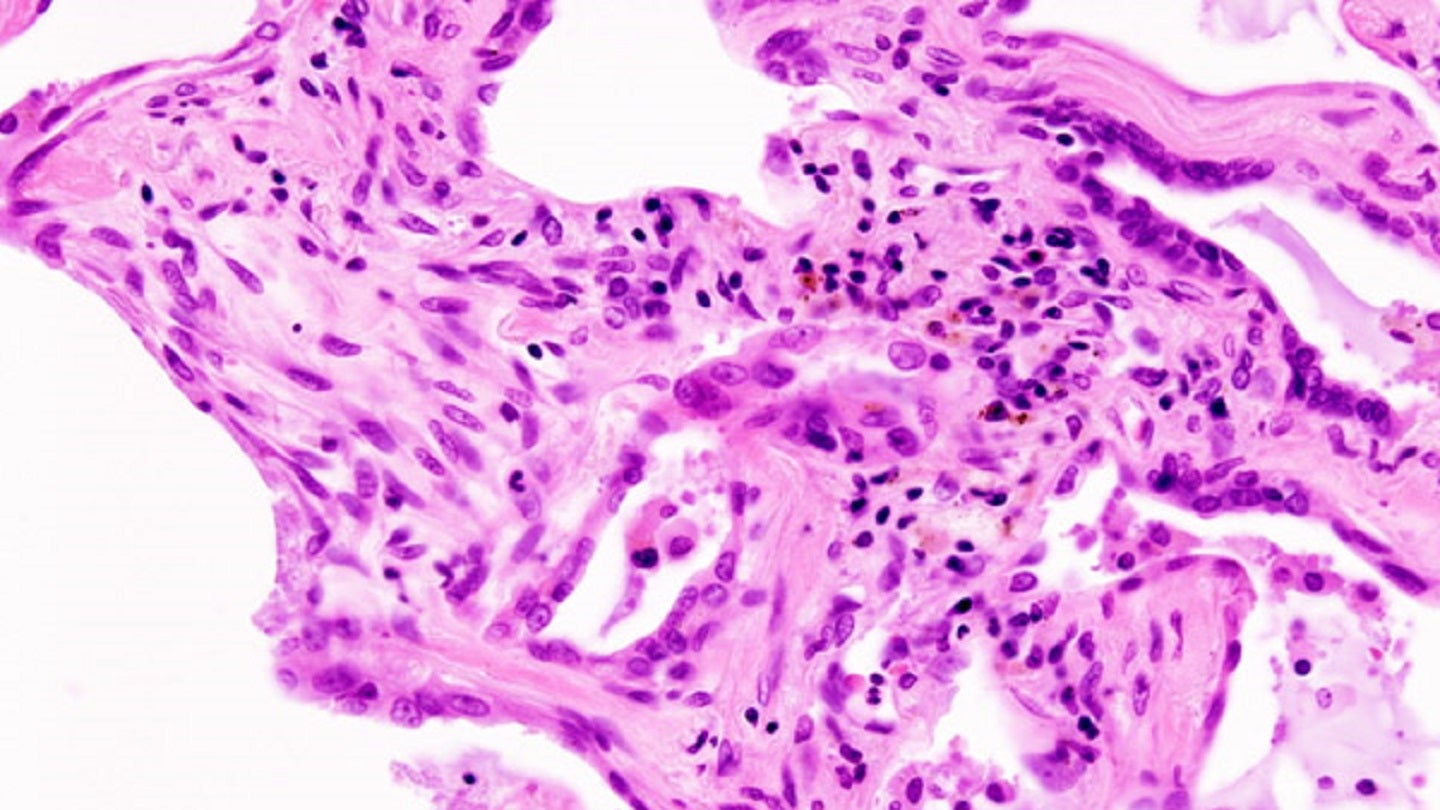
Cumberland Pharmaceuticals has announced plans to launch the Phase II FIGHTING FIBROSIS trial of oral ifetroban for idiopathic pulmonary fibrosis (IPF).
The company’s latest move follows approval from the US Food and Drug Administration for its investigational new drug application for the therapy.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The placebo-controlled, multicentre, double-blind, FIGHTING FIBROSIS study will assess the safety and efficacy of oral ifetroban in patients with IPF, a progressive interstitial lung disease.
It intends to enrol nearly 128 patients in more than 20 medical centres of excellence across the US.
Subjects will be block randomised, according to background therapy of pirfenidone or nintedanib. They will either receive 250mg of oral ifetroban once daily or a placebo for 52 weeks.
Lung function improvement as measured by the FVC in IPF patients on ifetroban compared to a placebo over 52 weeks is the primary objective of the study.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataCumberland Pharmaceuticals CEO AJ Kazimi said: “We are pleased that the FDA has cleared this new clinical programme as we work to develop new medicines for the future, especially those that address unmet medical needs.
“Given the exciting preclinical data demonstrating ifetroban can prevent lung fibrosis, we are very excited to advance directly to a Phase II study for IPF patients.”
As a potent and selective thromboxane-prostanoid receptor antagonist, Ifetroban exhibits anti-platelet, anti-vasospastic, antifibrotic, and antibronchospastic activities.
Cumberland is also planning to sponsor the FIGHT DMD Phase II study for assessing two oral ifetroban doses for treating cardiomyopathy associated with Duchenne muscular dystrophy.





