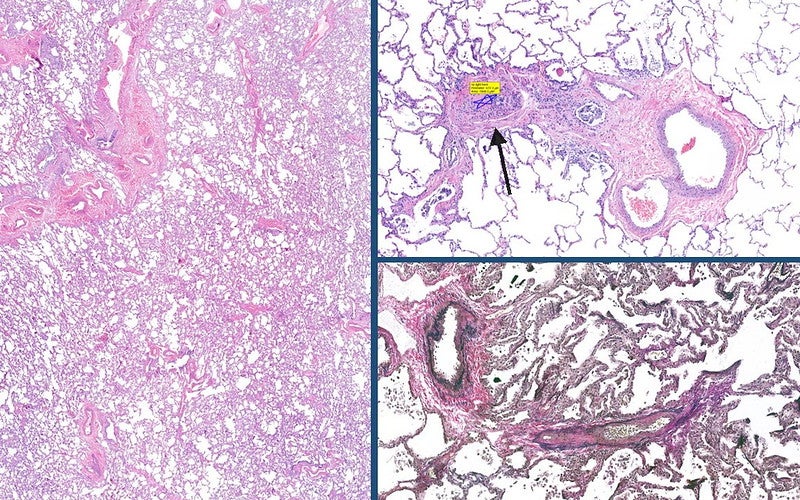
Daewoong Pharmaceutical announced that the first participant has been administered in the Phase II clinical trial of Bersiporocin to treat idiopathic pulmonary fibrosis.
The multinational Phase II clinical trial has been designed for assessing Bersiporocin’s safety and efficacy in changing the improvement rate of forced vital capacity (FVC) after receiving Bersiporocin or a placebo for 24 weeks.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It has been performed by nearly 30 institutions in the US and Korea and a total of 102 participants have been enrolled in the trial.
The company aims to complete the administration of Bersiporocin to the participants and evaluate the results by next year.
It intends to prove the antifibrotic efficacy of Bersiporocin and demonstrate the improvement of pulmonary function in the oncoming multinational Phase II clinical administration.
If successful, Bersiporocin will be further evaluated to treat many rare fibrosis diseases.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataUSC Los Angeles Keck School of Medicine Clinical Medicine professor Toby Maher said: “We are working hard to develop a new treatment for patients with idiopathic pulmonary fibrosis.
“The Phase II clinical trial of Daewoong Pharmaceutical’s Bersiposocin represents an important step in developing treatments that address the shortcomings of existing anti fibrotic therapies.”
Bersiposocin is a first-in-class candidate substance that is effective against idiopathic pulmonary fibrosis, a lung disease that is caused by overly formed fibrous tissue and hardening of the lungs.
Daewoong Pharmaceutical stated that it will continue to develop treatment for the fibroid lung patients, and extend the target to other progressive rare fibrosis diseases of the liver, skin, heart, lung, and kidney.
An official from the company stated: “Daewoong Pharmaceutical has set the significant first step to the patients by registering the first candidate and completing the Phase II clinical administration of Bersiporocin, the First-in-class treatment which is under self-development.”





