Early phase clinical research organisation Dr Vince Clinical Research (DVCR) has formed a strategic alliance with Clario for delivering cardiac assessments in clinical trials.
The strategic partnership will extend access to the Early Precision QT (EPQT) methodology of Clario.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
This enables biopharmaceutical firms to secure precise and cost-efficient cardiac safety data in the early stages of clinical development.
Clario’s technology platform combines eCOA, cardiac safety, medical imaging, precision motion, and respiratory endpoints.
The safety data is expected to meet International Council for Harmonization (ICH) E14 regulatory requirements and qualify sponsor companies for a thorough QT study waiver.
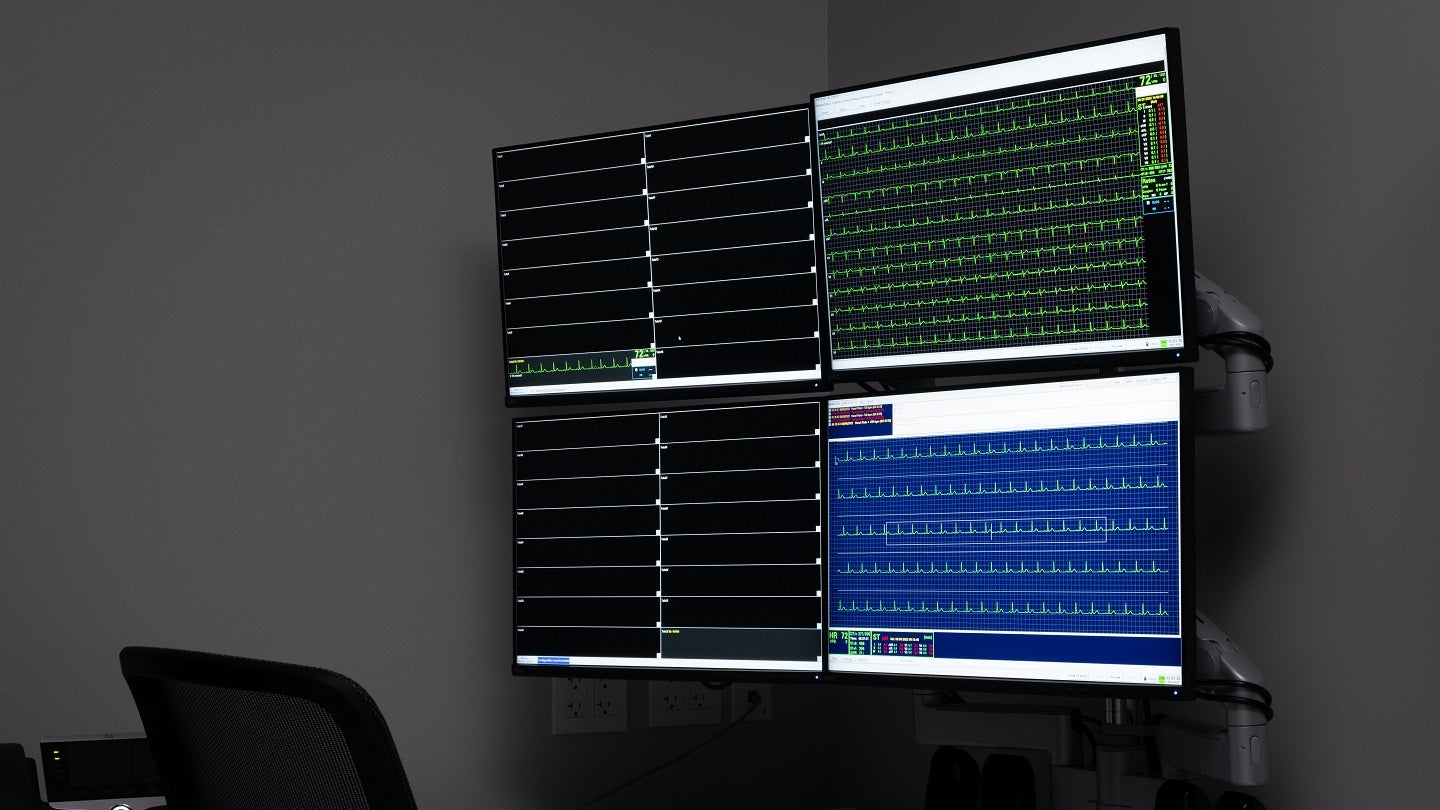
In this partnership, DVCR will use the technology with a Wi-Fi-enabled 12-lead telemetry system. This system archives data on-site and directly uploads it to the Cloud-based portal of Clario.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe data will then be used by site staff who can remotely monitor tracing quality thereby facilitating reduced data collection interruptions and improving the experience of study volunteers.
Clario Cardiology chief science and regulatory advisor Robert Kleiman said: “It was quickly obvious that the clinical pharmacology unit built by Dr Vince Clinical Research is exceptionally well-suited to conduct these complex trials given the site’s integrated technologies, state-of-the-art telemetry system, and deep clinical experience.
“Their organisation is already skillfully leveraging Clario’s platforms to provide high-quality cardiac data to our clients.
“The collected data was analysed by Clario’s experts to ensure that each extraction timepoint meets their robust standards for data quality and integrity. As a result, two of DVCR’s personnel were designated as Site Champions.”





