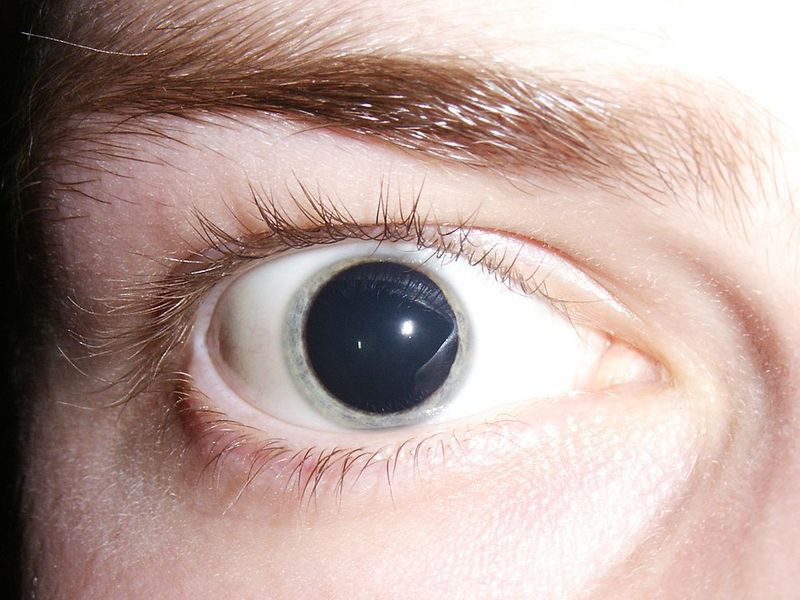
Eyenovia has enrolled the first patient in a Phase III trial for its MicroStat programme studying pharmacologic mydriasis.
The safety and efficacy of Eyenovia’s first-in-class fixed-combination of phenylephrine 2.5% and tropicamide 1% ophthalmic solution will be investigated as part of the MIST-1 and MIST-2 trials.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Pharmacologic mydriasis is essential for the standard dilated retinal fundoscopic examination and is said to be a key part of an estimated 80 million office-based comprehensive and diabetic eye exams performed annually in the US.
The solution will be administered as a micro-dose using the Optejet dispenser.
Eyenovia CEO and chief medical officer Dr Sean Ianchulev said: “With the enrollment of the first patient in the first pivotal trial of MicroStat for pharmacologic mydriasis, Eyenovia officially transitions into a Phase III company.
“We believe that our fixed-combination of phenylephrine and tropicamide has the potential to significantly improve both the patient experience and physician workflow during eye exams.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe randomised, double-blind, multi-centre Phase III trials will enrol approximately 65 participants each.
Treatment will be carried out on both eyes of participants on separate days using Eyenovia’s fixed combination mydriatic solution and each of the component solutions in the MIST-1 study.
Participants will receive a fixed combination mydriatic solution and a placebo on separate days for the MIST-2 study.
Mean change in baseline pupil diameter at 35 minutes after administration of study treatment will be the primary endpoint for both studies.
Topline results from both trials are expected to be announced in the first half of next year.





