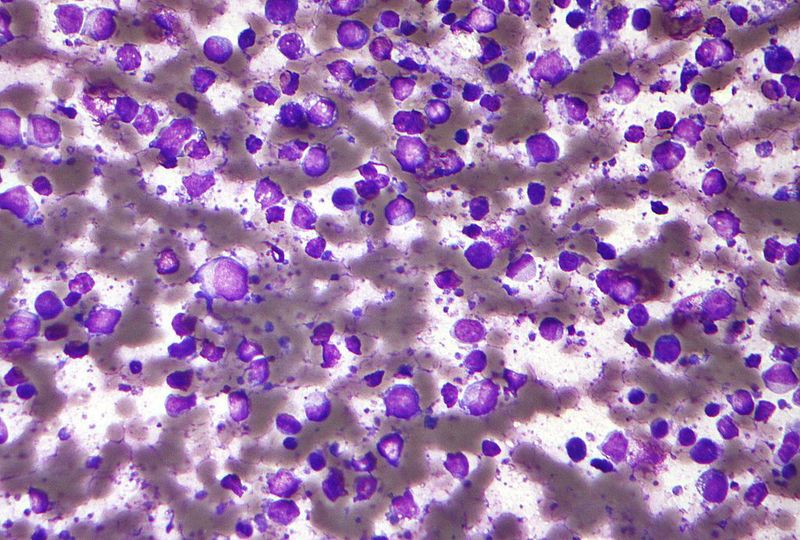
Immuno-oncology company Forty Seven has partnered with AstraZeneca’s Acerta Pharma to study a combination therapy for the treatment of diffuse large B-cell lymphoma (DLBCL).
The companies will conduct a clinical trial that will involve adjuvant administration of Forty Seven’s CD47 antibody 5F9 with rituximab and Acerta’s Calquence (acalabrutinib). Acerta will sponsor the trial.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
5F9 is an investigational monoclonal antibody designed to target CD47 and prevent recognition of CD47 by the SIRPα receptor on macrophages. This is intended to block the ‘don’t eat me’ signal used by cancer cells to escape ingestion by macrophages.
Initially, the compound is being developed to treat solid tumours, acute myeloid leukaemia, non-Hodgkin’s lymphoma (NHL), ovarian cancer and colorectal cancer.
Acalabrutinib is a Bruton tyrosine kinase (BTK) inhibitor. BTK signalling is known to activate pathways required for B-cell proliferation, trafficking, chemotaxis, and adhesion.
The new trial will build upon the data obtained from a Phase Ib clinical trial of 5F9 and rituximab combination in patients with NHL. The latest triple combination is intended to further optimise the therapy for DLBCL, which is an aggressive form of NHL.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataForty Seven chief business officer Craig Gibbs said: “We are pleased to enter this collaboration with Acerta, which expands the breadth of our 5F9 development programme to include an additional triplet regimen, with the potential to offer patients a treatment option that is more easily administered and the benefit of multiple distinct approaches to treating cancer.”
5F9 has the US Food and Drug Administration (FDA) fast track designation in relapsed or refractory diffuse large B-cell lymphoma and follicular lymphoma, two types of B-cell non-Hodgkin’s lymphoma.
In October 2017, the FDA awarded accelerated approval for Calquence to treat adults suffering mantle cell lymphoma who have received at least one prior therapy.





