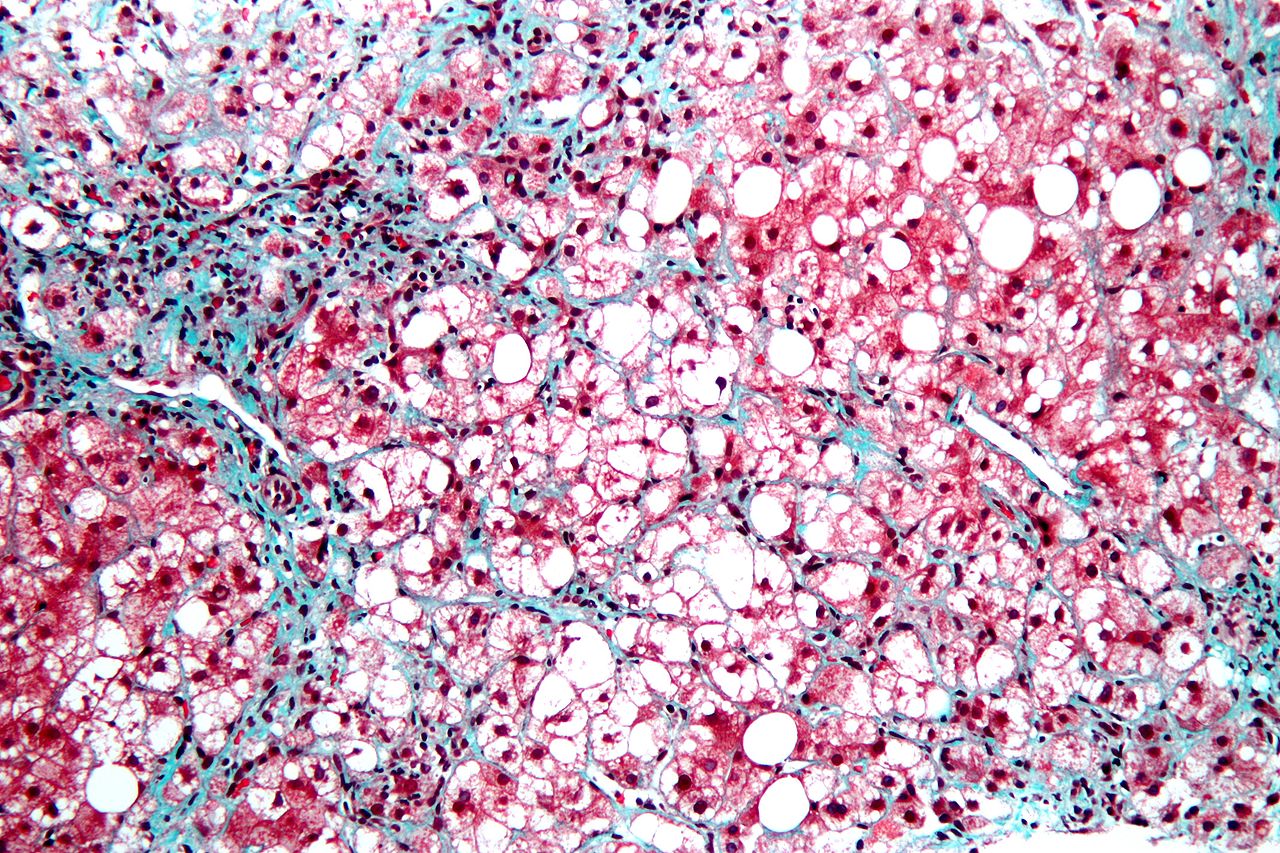
Gilead Sciences and Novo Nordisk have announced positive results from a Phase II proof-of-concept trial evaluating combinations of Novo Nordisk’s semaglutide with Gilead’s cilofexor and/or firsocostat in participants with non-alcoholic steatohepatitis (NASH).
The trial showed that in people with NASH and mild to moderate fibrosis, all regimens were well tolerated, meeting its primary endpoint.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Semaglutide is a GLP-1 receptor agonist, while cilofexor and firsocostat are Gilead’s investigational FXR agonist and ACC inhibitor, respectively.
The five-arm trial analysed the drug combinations over 24 weeks in 108 people with NASH.
Post-hoc analyses showed statistically significant improvements in hepatic steatosis and liver injury in the combination arms as compared to semaglutide alone at 24 weeks, meeting exploratory efficacy endpoints of the trial.
Statistically significant differences in liver stiffness were not noticed between different arms, even though a decline was observed in all groups.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataGilead Sciences Inflammation Clinical Development senior vice-president Mark Genovese said: “Gilead is focused on delivering scientific advances that can improve the lives of people with liver disease, both through our own innovation and in partnership with companies with complementary expertise, such as Novo Nordisk.
“These data offer new insights into potential therapeutic approaches to treating NASH, a disease which currently has limited treatment options.”
The latest data is backed by results from the preclinical murine model, which analysed the combination drug for treating NASH.
Data showed that semaglutide significantly improved NASH and fibrosis-related endpoints, while the drug plus cilofexor and/or GS-834356, an analogue of firsocostat, improved liver fat reduction.
A chronic and progressive liver disease, NASH is characterised by fat accumulation and inflammation in the liver, leading to scarring or fibrosis that impairs liver function.





