Hoth Therapeutics has announced the formation of its subsidiary, Hoth Therapeutics Australia, in preparation for upcoming clinical studies.
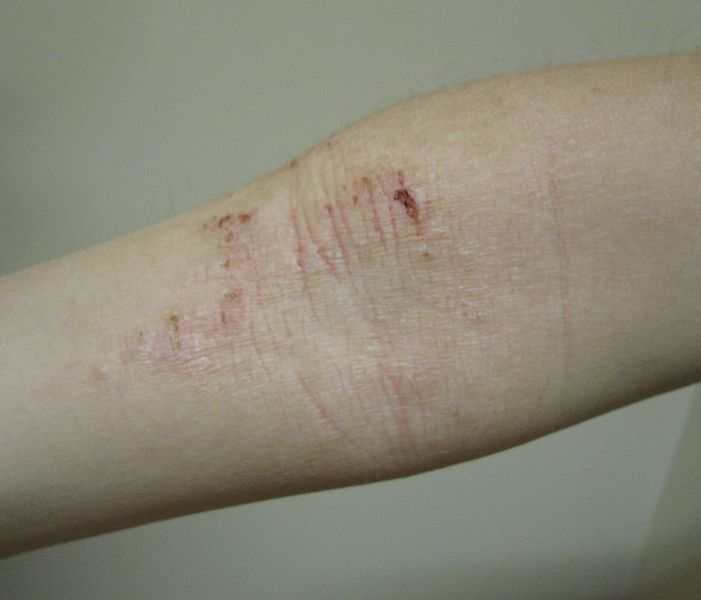
The clinical trials will be focused on providing therapeutics for atopic dermatitis, also known as eczema, which is a dermatological disorder.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The newly formed subsidiary will oversee the preparation and execution of Hoth’s first clinical trial, which examines the efficacy and safety of BioLexa indicated for the treatment of mild-to-moderate atopic dermatitis.
The entity will also be eligible for a significant research and development (R&D) tax rebate.
It will supervise data management, medical monitoring, quality insurance, regulatory and central laboratory services to support the clinical trial of the therapeutic BioLexa.
Hoth Therapeutics CEO Robb Knie said: “We are excited by the opportunity to work with Australian researchers and members of the international pharmaceutical industry, in regards to the development of our proprietary atopic dermatitis treatment.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“This endeavor is a significant milestone for us, as we move towards clinical trials. Hoth is committed to working diligently with physicians, and regulators in order to successfully pilot our BioLexa Platform to the millions of people that are afflicted with atopic dermatitis.”
The new entity was formed in connection with full-service contract research organisation Novotech and financial solutions provider CoSec Consulting. Both the companies are based in Australia.
Nevada-based Hoth Therapeutics is a biopharmaceutical company focused on unique targeted therapeutics for patients suffering from atopic dermatitis. It has exclusive worldwide rights to the BioLexa Platform.
The BioLexa Platform is a proprietary and patented antimicrobial therapeutic based on cutting-edge scientific research from University of Cincinnati.





