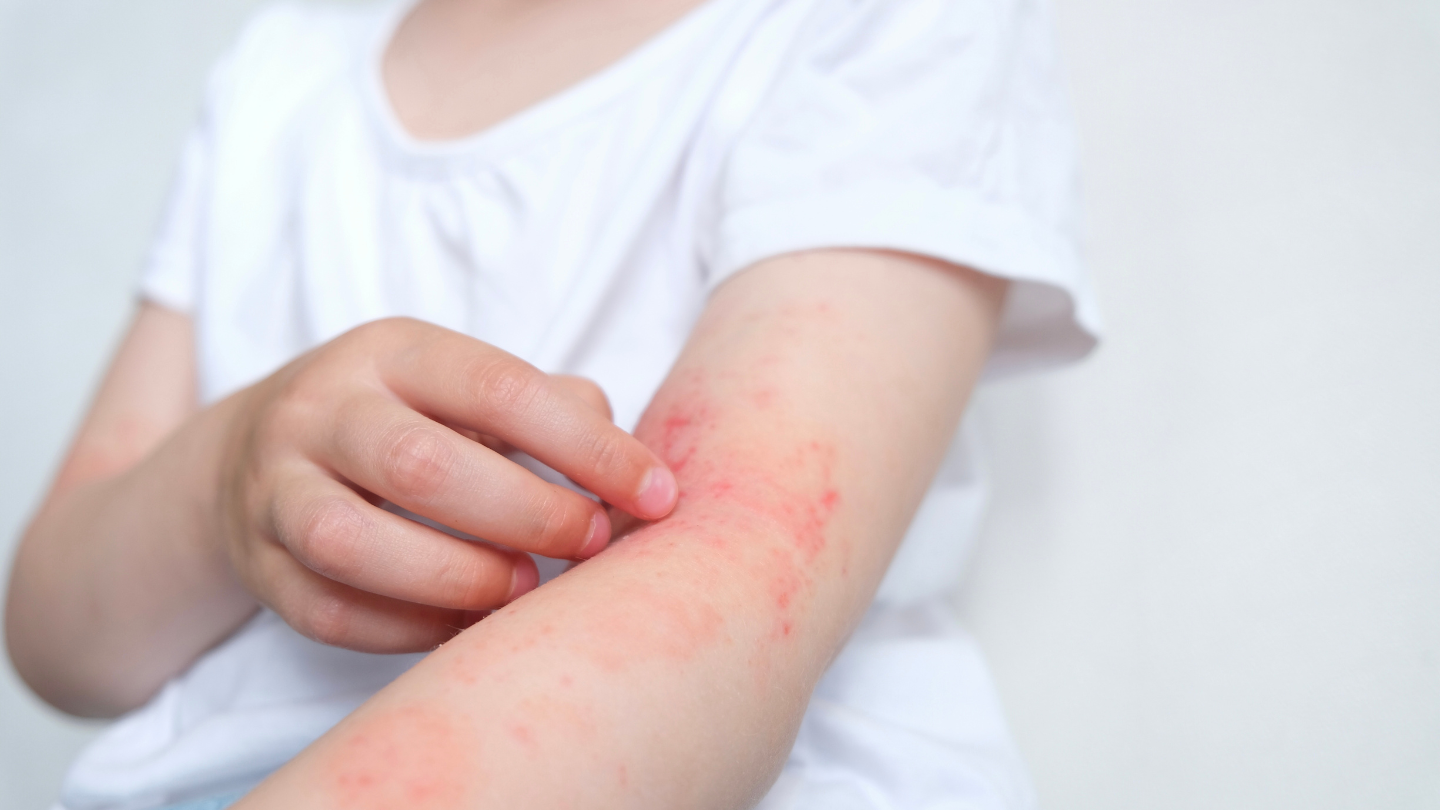
Inmagene Biopharmaceuticals has started a proof-of-concept trial of the pipeline drug IMG-007 in adult patients with moderate-to-severe atopic dermatitis (AD).
The clinical stage biotechnology company based in Shanghai has dosed the first patient in a Phase I/II global multicentre proof-of-concept (POC) study (NCT05984784) of IMG-007. The study will assess the safety, pharmacokinetics, and efficacy of IMG-007 in AD patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
In a single ascending dose study in healthy adults which has already been completed, IMG-007 demonstrated a half-life that exceeded the average for conventional IgG antibodies. A target serum concentration is maintained for approximately 12 to 18 weeks after a single dose which allows patients to have longer drug holidays. IMG-007 is the lead compound in development by Inmagene.
Chief medical officer of Inmagene, Yufang Lu said: “IMG-007 represents an investigational drug with multiple potential indications. We are excited to begin this trial of IMG-007 in AD patients, following favourable safety and highly differentiated pharmacokinetic results of the earlier trial.
“We are continuing our evaluations of IMG-007’s potential in other diseases where OX40 signalling pathway plays an important pathogenic role.”
What is IMG-007
IMG-007 is a humanised IgG1 monoclonal antibody (mAb) that specifically binds to the OX40 receptor. OX40-OX40L axis is important in T cell activation, expansion, and survival. In nonclinical studies, Inmagene reports that IMG-007 potently and completely blocked signalling between OX40 and OX40L. The Fc region of the drug has been bioengineered, resulting in an extended half-life and a silenced antibody-dependent cell-mediated cytotoxicity (ADCC) function.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataOther pipeline drugs
Inmagene is developing three other clinical-stage drug candidates, IMG-004, IMG-008 and IMG-020. IMG-004 is a non-covalent, reversible BTK inhibitor being trialled in patients with multiple sclerosis and completing Phase I clinical development.
IMG-008 is a long-acting mAb against IL-36R and is entering global Phase I clinical development. It is being trialled in patients with hidradenitis suppurativa and generalised pustular psoriasis.
Finally, IMG-020, also referred to as izokibep, is an anti-IL-17 small protein therapeutic in global clinical development for a number of indications including hidradenitis suppurativa, psoriatic arthritis, uveitis, psoriasis and axial spondyloarthritis.



