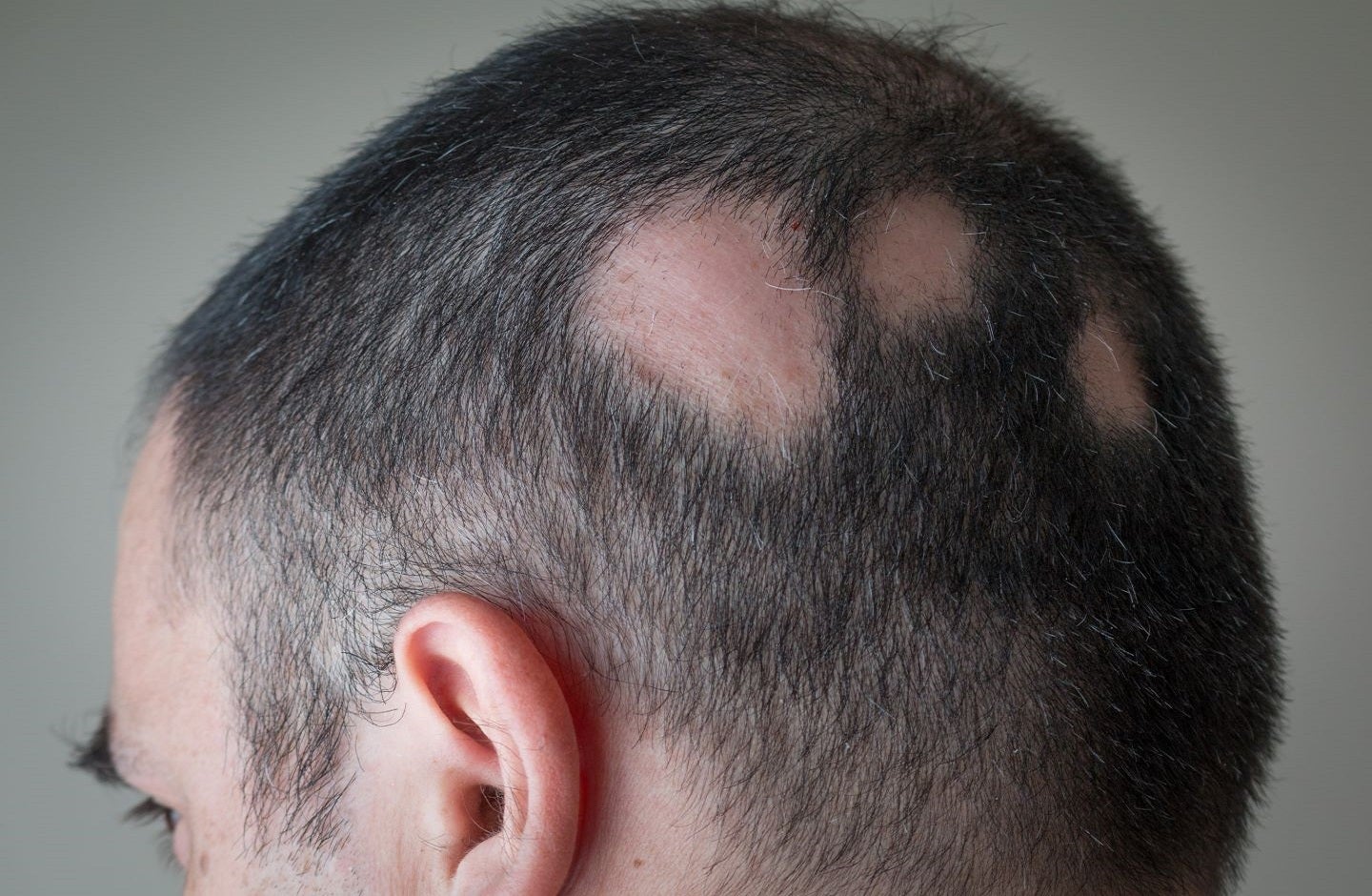
Inmagene Biopharmaceuticals has dosed the first subject in a Phase IIa trial of IMG-007, an anti-OX40 monoclonal antibody (mAb) with an extended half-life, to treat alopecia areata (AA).
The global multicentre trial will assess the efficacy, safety, biomarkers, and pharmacokinetics of IMG-007 in AA patients with 50% or greater scalp hair loss.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The humanised IgG1 mAb particularly attaches to the OX40 receptor as well as designed to potently block the signalling between OX40 and its ligand.
IMG-007’s extended half-life enables less frequent dosing while its silenced antibody-dependent cell-mediated cytotoxicity (ADCC) function cuts safety risks.
It has shown a favourable safety profile, without any pyrexia or chill reports, in a Phase I study in healthy adult participants.
A single IMG-007 treatment at projected therapeutic dose levels maintained the desired exposure for a duration of 12 to 18 weeks, potentially enabling IMG-007 to be administered every 12 weeks.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIt is also being assessed in a global Phase IIa study in adults with atopic dermatitis (AD) with an interim data readout anticipated in the second quarter of next year.
Inmagene CEO Jonathan Wang said: “Alopecia areata is a devastating disease which affects approximately 147 million people globally. Currently, there are limited treatment options and no approved biologics for AA.
“IMG-007 could potentially provide a safe and effective biologic therapy with once every 12 weeks dosing regimen for AA patients.”
AA is a chronic autoimmune condition causing hair loss without scarring, which can affect the scalp, face, and body.





