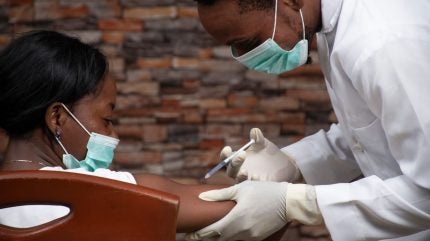
Subjects at HJF Medical Research International in Nigeria have received a Lassa fever virus (LASV) vaccine candidate in the first Phase II study sponsored by the International AIDS Vaccine Initiative (IAVI).
Dubbed IAVI C105/PREVAIL15, the trial is funded by the Coalition for Epidemic Preparedness Innovations (CEPI).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It will assess the safety, tolerability, and immunogenicity of varying dosages of the vaccine in adults, including those living with HIV, as well as in adolescents and children aged two years and above.
Approximately 612 subjects will be enrolled in Liberia, Ghana and Nigeria. They will be monitored for six months to evaluate safety and immune responses, with a subset followed for an additional two years.
The study design involved collaboration with Nigerian health authorities, including the Nigeria Centre for Disease Control and Prevention and the Nigeria Lassa Vaccine Taskforce.
IAVI plans to expand the trial to include more subjects from the Noguchi Memorial Institute for Medical Research, the University of Ghana, and the Partnership for Research on Vaccines and Infectious Diseases in Liberia, subject to regulatory approvals.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataBatavia Biosciences manufactured the LASV vaccine candidate in the Netherlands. The vaccine utilises the same recombinant vesicular stomatitis virus vector platform as the licensed Zaire ebolavirus vaccine of Merck, ERVEBO.
Previous trials in the US and Liberia have demonstrated that the LASV vaccine candidate is well-tolerated and capable of inducing a robust immune response. These immune responses were sustained for up to a year post-vaccination.
IAVI emerging infectious diseases and epidemiology vice-president and head Swati Gupta said: “Continued outbreaks of Lassa fever and the emergence of Ebola Sudan in Uganda both underscore the need to have vaccines for known disease threats available for evaluation and use during outbreak situations, the overarching goal of IAVI’s emerging infectious disease programme.
“IAVI C105 is an important step toward attaining eventual licensure of a Lassa fever vaccine, should IAVI’s vaccine candidate prove to have an acceptable safety profile and be efficacious.”



