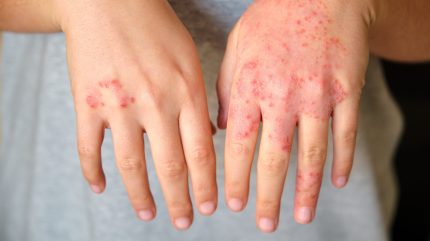
LEO Pharma has reported positive outcomes from the 16-week interim analysis of the randomised Phase IIIb ADHAND trial of fully human biologic, tralokinumab, to treat adults with moderate-to-severe atopic dermatitis (AD) on the hands.
The double-blind, randomised, placebo-controlled trial is intended for these patients who are candidates for systemic therapy.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Tralokinumab targets the interleukin-13 (IL-13) cytokine, a primary contributor to the symptoms of AD.
The trial aims to assess the safety and efficacy of tralokinumab 300mg administered biweekly as a single agent.
Subjects were randomised and given either the therapy or a placebo.
The interim analysis focused on the primary endpoint, the proportion of subjects achieving a score of 0 or 1 on the Investigator’s Global Assessment for atopic dermatitis on the hands (IGA-AHE).

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAccording to the company, key secondary endpoints included decreases in itch and pain scores of ≥4, measured by the Hand Eczema Symptom Diary (HESD) from baseline, and improvements on the Hand Eczema Severity Index (HECSI).
The key safety endpoint of the study was the number of treatment-emergent adverse events from baseline to week 16.
The primary goal of the trial was met, with improvement in AD on the hands post 16 weeks of tralokinumab treatment versus the placebo.
This therapy was found to be tolerated well, with most adverse events being non-serious and of mild or moderate severity.
The trial will continue to week 32, with final outcomes anticipated by the year’s end.
The company noted that after the 16-week analysis, all subjects will enter a 16-week open-label treatment period where they will be given the injections of tralokinumab every two weeks.
LEO Pharma science, search and innovation executive vice-president and chief scientific officer Dr Jacob Pontoppidan Thyssen said: “These interim results mark an important step forward in addressing the needs of patients with moderate-to-severe atopic dermatitis that affects high-burden and hard-to-treat areas such as the hands despite available treatments.”
In May 2025, LEO Pharma reported positive topline outcomes from the Phase IIb trial of temtokibart, a potential new treatment for the adult population with moderate-to-severe AD.





