Life science company Micron Medical has published positive results from a Phase I/II study evaluating its measles and rubella microneedle patch (MNP) showing that the needle-free vaccine demonstrates safety and immunogenicity.
The data, published in The Lancet, demonstrates that samples from 93% of infants showed seroconversion for measles and 100% seroconverted for rubella following measles rubella vaccine (MRV)-MNP administration. In comparison, with the MRV subcutaneous (SC) injection, 90% and 100% of patient samples showed seroconversion for measles and rubella respectively. Seroconversion refers to the development of antibodies against a particular pathogen in the blood.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The trial enrolled 45 adults, 120 toddlers (15–18 months), and 120 infants (9–10 months), who were randomly allocated and vaccinated with either an MRV-MNP vaccine patch and a placebo SC injection, or a placebo-MNP and an MRV SC injection. There were no related severe or serious adverse events reported from the study. Micron plans to evaluate its technology in additional clinical trials within the next 12 months.
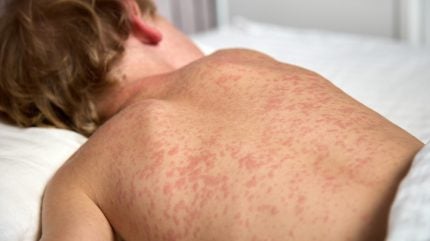
Measles and rubella are both highly contagious viral infections. Measles primarily affects children, causing a red rash, fever, and runny nose. Rubella typically presents with a mild fever and rash but poses significant risks to pregnant women, potentially causing miscarriage or birth defects.
In 2022, there were an estimated 136,000 measles deaths globally, mostly among unvaccinated or under vaccinated children under the age of 5 years, according to the World Health Organization (WHO). The US Centers for Disease Control and Prevention reported that over 64 cases were detected in the US by March this year, which exceeded the total number of cases noted in the previous year.
The needle-free vaccine has been developed using Micron’s microarray technology. This has been identified as the highest global priority innovation for overcoming immunisation barriers in low and middle-income countries by the Vaccine Innovation Prioritisation Strategy (VIPS) Alliance – a consortium between the WHO, GAVI, UNICEF, PATH, and the Bill & Melinda Gates Foundation, as per the 1 May announcement.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataLast year, the Bill & Melinda Gates Foundation granted Micron a $23.6m grant to fund mass production of Micron’s needle-free vaccines and enable commercialisation once approved by the appropriate regulatory authorities, following an additional clinical study.
In the announcement accompanying the data, Micron’s CEO Steven Damon said: “Making injectable pharmaceuticals available without needles has the potential to eradicate disease in low- and middle-income countries as well as to make treatments and vaccines more accessible in developed nations and among military personnel.”





