The US National Institutes of Health unit National Eye Institute (NEI) has reported that a Phase II clinical trial of the drug minocycline for the treatment of dry age-related macular degeneration (AMD) did not show any benefit.
The oral antibiotic, known for its anti-inflammatory properties, could not decelerate vision loss or geographic atrophy expansion in trial participants.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
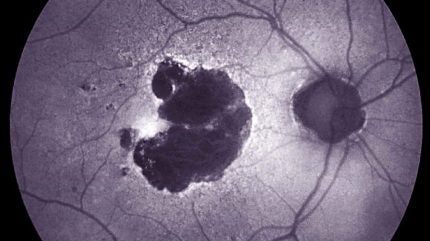
Dry AMD is a condition impacting the macula, a crucial part of the retina responsible for clear central vision, where inflammation, particularly by microglia, is believed to play a role in disease progression.
Patches of light-sensing photoreceptors and the support cells that surround them die off in dry AMD patients, leaving behind what is known as geographic atrophy.
As these zones grow, people gradually lose more of their centre vision. Immune cells called microglia help preserve tissue and remove waste.
The trial, funded by the NEI Intramural Program and partly conducted at the NIH Clinical Center, sought to determine if minocycline could hinder microglia and consequently slow the geographic atrophy progression and associated vision loss in dry AMD patients.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIt enrolled 37 subjects aged 55 years and above with GA in at least one eye.
These patients were enrolled at two sites: the NIH Clinical Center in Bethesda, Maryland, and the Bristol Eye Hospital in the UK.
After monitoring the rate of geographic atrophy expansion over a nine-month baseline period, the trial subjects were given a twice-a-day dose of minocycline for two years.
Researchers then assessed the rate of geographic atrophy expansion during the minocycline treatment phase against the baseline rate.
The findings revealed no significant difference in the rate of atrophy expansion or the progression of vision loss while on minocycline.
These results suggest that minocycline, despite prior studies indicating its potential to lower inflammation and microglial activity in the eye, including the retina, does not confer the expected benefits for dry AMD.
Minocycline has demonstrated positive effects in managing other conditions such as diabetic retinopathy.





