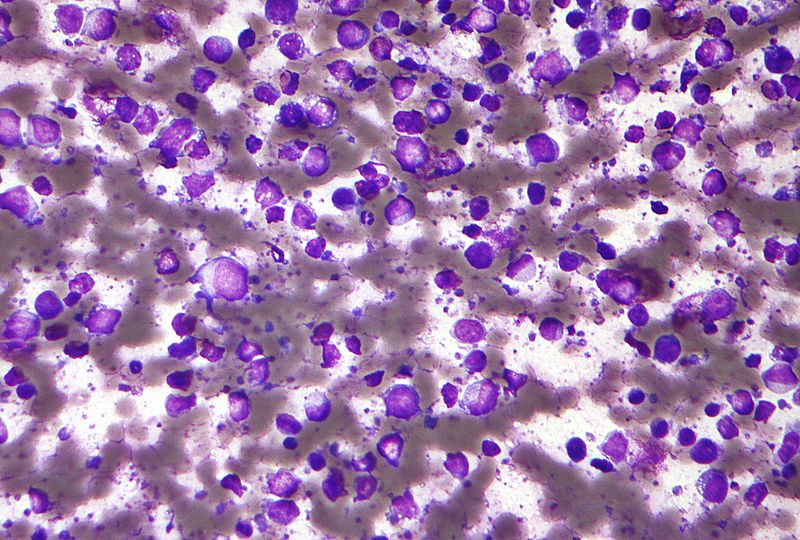
MorphoSys’ ongoing single-arm Phase II clinical trial of tafasitamab (MOR208) in combination with lenalidomide to treat relapsed or refractory diffuse large B cell lymphoma (r/r DLBCL) has met its primary endpoint.
The L-MIND trial enrolled a total of 80 patients with r/r DLBCL who are ineligible for high-dose chemotherapy (HDC) and autologous stem cell transplantation (ASCT).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The subjects were given tafasitamab and lenalidomide and followed-up for at least one year.
MorphoSys noted that the patients had an average age of 72 years and received a median of two prior treatment lines.
MorphoSys chief development officer Dr Malte Peters said: “We are very pleased by the results from the primary analysis of the L-MIND study and are especially encouraged by the durability of the responses and the OS that we are seeing.
“If approved, we believe that with tafasitamab in combination with lenalidomide we can offer a chemo-free treatment option to patients with r/r DLBCL who are ineligible for HDC and ASCT.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAs part of the L-MIND trial, the investigational humanised Fc-enhanced monoclonal antibody tafasitamab was investigated in combination with lenalidomide in patients with r/r DLBCL.
Tafasitamab, which is directed against CD19, is currently in clinical development in blood cancer indications.
The primary endpoint is defined as the best objective response rate (ORR). The ORR was 60% and the complete response (CR) rate was 43%.
In the trial, efficacy parameters, such as response rates, showed comparable results in most patient subgroups of interest.
The company plans to complete a BLA submission to the FDA by the end of this year.





