
A new clinical trial conducted by the University of California, San Francisco (UCSF) in five children with Dravet syndrome showed promising results similar to those found in a genetic zebrafish model.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Dravet syndrome is a severe form of pediatric epilepsy caused by a genetic mutation, which leads to frequent daily drug-resistant seizures and developmental delays.
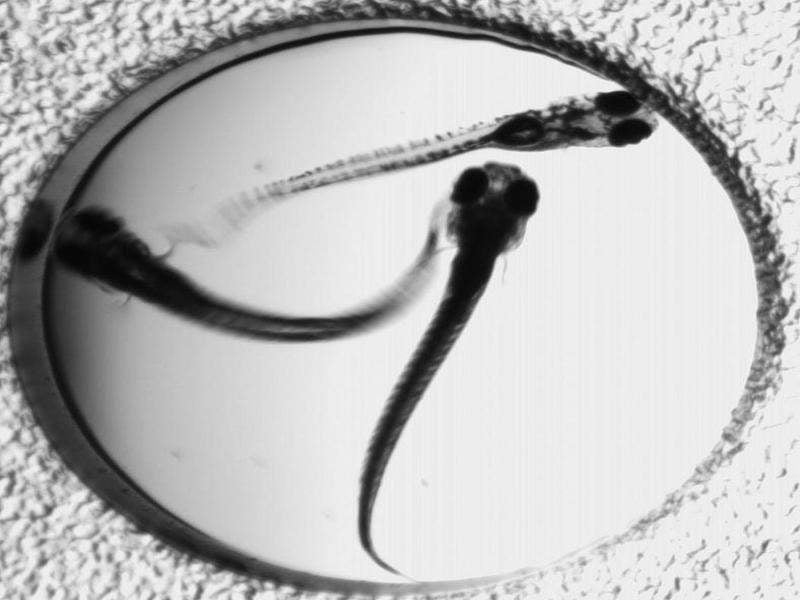
Funded by the National Institutes of Health's (NIH) division national institute of neurological disorders and stroke (NINDS), the drug was initially tested in a zebra-fish model that was infused with the genetic mutation to cause epilepsy.
It was found that lorcaserin suppressed seizure activity in the fish. The drug was then studied via a compassionate use, off-label programme in children who were resistant to other anti-epileptic drugs.
NINDS programme director Vicky Whittemore said: “This is the first time that scientists have taken a potential therapy discovered in a fish model directly into people in a clinical trial.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“These findings suggest that it may be possible to treat neurological disorders caused by genetic mutations through an efficient and precision medicine-style approach.”
The clinical trial results during the first three months of treatment showed that one of the patients suffering with multiple seizures every day, was seizure-free for two weeks.
The data after the intial three months indicated an increase in seizure activity while the frequency was less, compared to the beginning of the trial.
While some children experienced a decrease in appetite, no severe side effects were observed.
The researchers claimed that using zebrafish can minimise the time between identification of a potential treatment and transferring it to individuals.
Image: A zebrafish model of Dravet syndrome, a severe form of pediatric epilepsy, may help scientists quickly find drugs for the devastating disease. Photo: courtesy of NIH.





