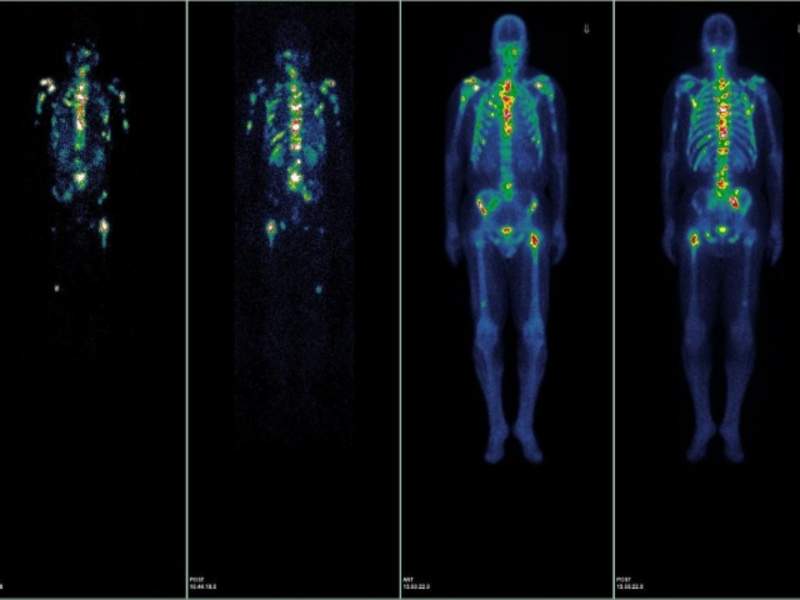

US-based university hospital NewYork-Presbyterian and Weill Cornell Medicine have commenced a Phase I trial to evaluate a small molecule called Lutetium 177Lu-PSMA-617 for the treatment of patients with metastatic prostate cancer in the country.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The small molecule is being used to target a protein called prostate-specific membrane antigen (PSMA), which is reportedly found in 85%-90% of metastasised prostate cancers.
Lutetium 177Lu-PSMA-617 binds to PSMA and delivers accurate radiation therapy to shrink the cancer, even in cases where cells have not yet formed a visible tumour, as observed on a bone or CT scan.
Designed to establish the maximum dose level of the drug that will have no significant side effects, the trial will include prostate cancer patients whose cancer has progressed beyond the prostate and is not responding to hormonal therapy.
NewYork-Presbyterian genitourinary oncology programme medical director and Weill Cornell Medicine hematology and oncology associate professor Dr Scott Tagawa said: “This trial represents a new frontier in the treatment of metastatic prostate cancer.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“While this type of therapy has shown promise, this is the first trial of its kind in the United States. So far, patients are doing well.”
A similar approach is currently in practice in Germany to treat metastatic prostate cancer patients who have exhausted standard treatment options.
The first monoclonal antibodies with the capacity to bind to prostate cancer cells’ PSMA were developed by Dr Neil Bander, an urological oncology professor at Weill Cornell Medicine and an urologic oncologist at NewYork-Presbyterian.
Image: Imaging after treatment with radiolabelled PSMA molecule. Photo: courtesy of Weill Cornell Medicine.





