Synta Pharmaceuticals has started a Phase II clinical trial (I-SPY 2 TRIAL) of its heat shock protein 90 (Hsp90) inhibitor, ganetespib, as a neoadjuvant therapy for patients with breast cancer.
Hsp90 is a molecular chaperone which controls the folding and activation of a number of client proteins that drive tumour development and progression.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The randomised, controlled, multicentre Phase II trial will evaluate ganetespib in women with newly diagnosed, locally advanced breast cancer that is designed to test whether adding investigational drugs to standard chemotherapy is better than standard chemotherapy alone in the neoadjuvant setting.
Sponsored by QuantumLeap Healthcare Collaborative, the trial is being conducted by a consortium, which includes the US Food and Drug Administration (FDA), National Cancer Institute (NCI), pharmaceutical companies, leading academic medical centres and patient advocacy groups under its umbrella.
The trial uses genetic or biological markers from individual patients’ tumours in order to screen new treatments, identify which treatments are most effective in specific patient subgroups.
The company said that regimens that have a high Bayesian predictive probability of showing superiority in a 300 patient Phase III confirmatory trial in at least one of ten predefined signatures may graduate from this Phase II I-SPY 2 trial.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSynta chief executive officer Anne Whitaker said: "The I-SPY 2 trial offers us the opportunity to leverage the encouraging data we observed in earlier studies to further advance the development of ganetespib in breast cancer in a well-regarded consortium-sponsored trial.
"We are very pleased that ganetespib was selected for this innovative study, which is redefining how promising investigational compounds are rapidly evaluated as potential new treatments for breast cancer.
"We continue to support this and several other randomised investigator-led studies of ganetespib in both solid and hematologic malignancies."
Initially, ganetespib will be available to patients with HER2 negative disease, with the intent to expand its eligibility to all breast cancer subtypes, including HER2 positive after safety testing with trastuzumab is completed.
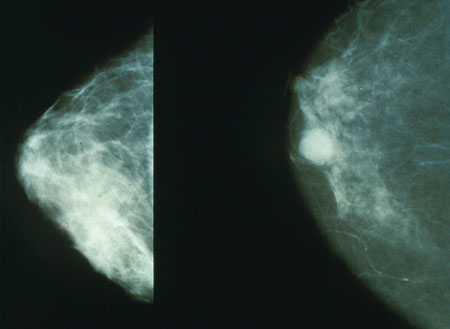
Image: Mammograms showing a normal breast (left) and a cancerous breast (right). Image: courtesy of Morning2k.





