
Valerion Therapeutics has started dosing patients in a Phase I/II clinical trial of VAL-1221 to treat patients suffering from late-onset Pompe disease.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
VAL-1221 is a fusion protein combining, recombinant human acid alpha-glucosidase (rhGAA) with the firm’s antibody-mediated delivery technology for increased intracellular delivery.
The dual uptake mechanism of VAL-1221 targets lysosomal, as well as extra-lysosomal glycogen, and is expected to improve glycogen clearance and patient outcomes.
The randomised, international, parallel active control, single-ascending and multiple-ascending dose escalation Phase I/II trial will investigate the safety, tolerability, pharmacokinetics, pharmacodynamics and preliminary efficacy of the drug candidate in approximately 12 subjects.
The trial is initially set to be performed at the Duke University Medical Centre, US, and the National Hospital for Neurology and Neurosurgery, UK, and will enrol ambulatory and ventilator-free patients who received prior Myozyme or Lumizyme therapy.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataValerion Therapeutics CEO Deborah Ramsdell said: "While current Pompe disease therapies are directed only to lysosomal glycogen, VAL-1221 has demonstrated in preclinical studies the potential to uniquely target and clear both lysosomal and extra-lysosomal glycogen in the cytoplasm.”
The exploratory efficacy endpoints of the trial include a six-minute walk test, pulmonary function testing, quantitative and qualitative muscle testing and patient-reported outcomes.
Patients who are found to be responding during the three month trial period will be eligible to enter an open-label extension study.
The top-line results from the Phase I/II trial are expected to be available in the fourth quarter of this year.
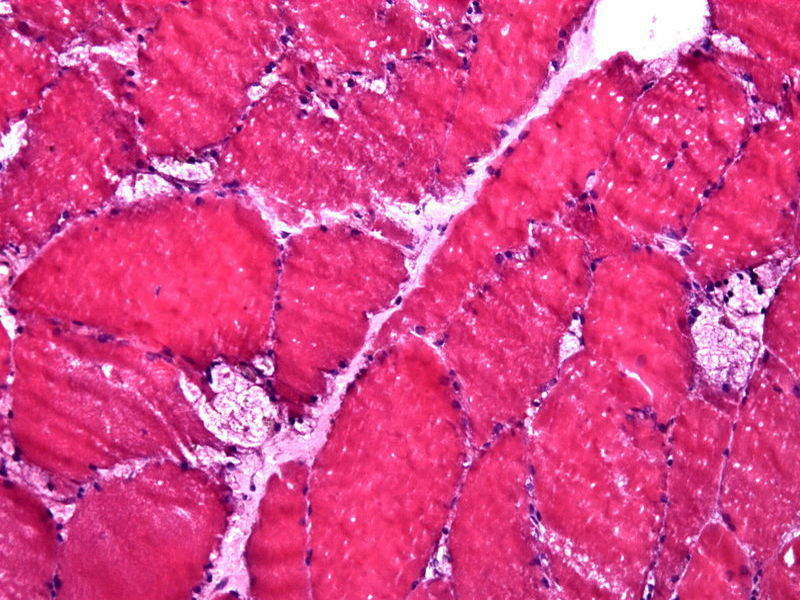
Image: Muscle biopsy showing large vacuoles in a case of Pompe disease. Photo: courtesy of Jensflorian.





