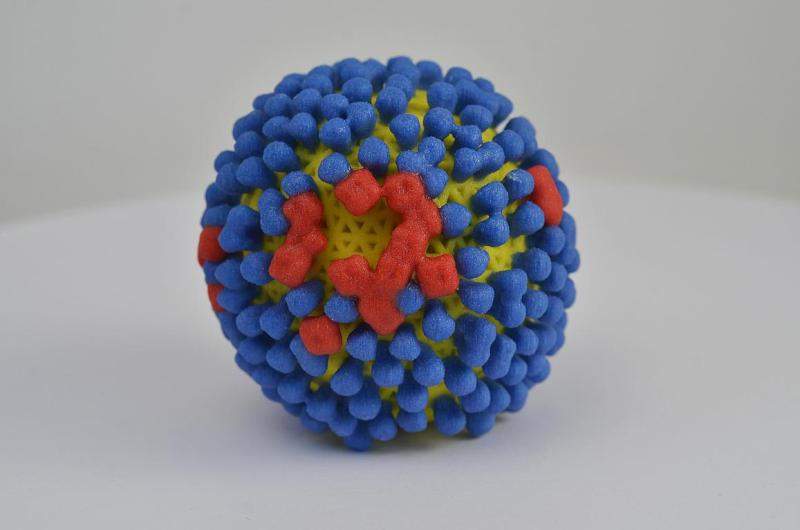
An early stage clinical trial, funded by the US National Institute of Allergy and Infectious Diseases (NIAID), has started enrolling subjects at a Vaccine and Treatment Evaluation Unit (VTEU) site at Saint Louis University, Missouri.
The Phase l trial is a double-blind, randomised, placebo-controlled study that aims to investigate the safety and immunogenicity of an intranasal influenza vaccine in 50 healthy subjects aged 9-17.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Administration of the investigational vaccine, developed by FluGen using a strain of seasonal influenza virus (H3N2), will be followed by licensed inactivated Quadrivalent Influenza Vaccine (QIV).
During the trial, half of the subjects will receive the nasal vaccine and the other half will receive a dose inactive saline (placebo) solution via nasal spray.
All subjects will be treated with the QIV vaccine three months after receiving the initial nasal vaccine or placebo.
The trial, which is led by principal investigator Daniel Hoft, aims to identify whether the combination of the licensed and experimental vaccine can offer protection against influenza viruses compared with the QIV vaccine alone.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataInvestigators of the trial will conduct various tests on the blood samples of the subjects at four time points after the first vaccination, as well as three weeks after the second vaccination.
The tests will be conducted to find evidence of immune responses from antibody-producing cells and from the cellular arm of the immune system.
NIAID director Anthony Fauci said: “We are hopeful that newer kinds of influenza vaccines, such as the candidate being tested in this trial, will provide protection even if their components do not precisely match the currently circulating influenza virus strains.”
Any individuals over six months of age are recommended to take an annual vaccination against influenza. However, because of the changing nature of the flu virus, vaccines are required to be reformulated annually.





