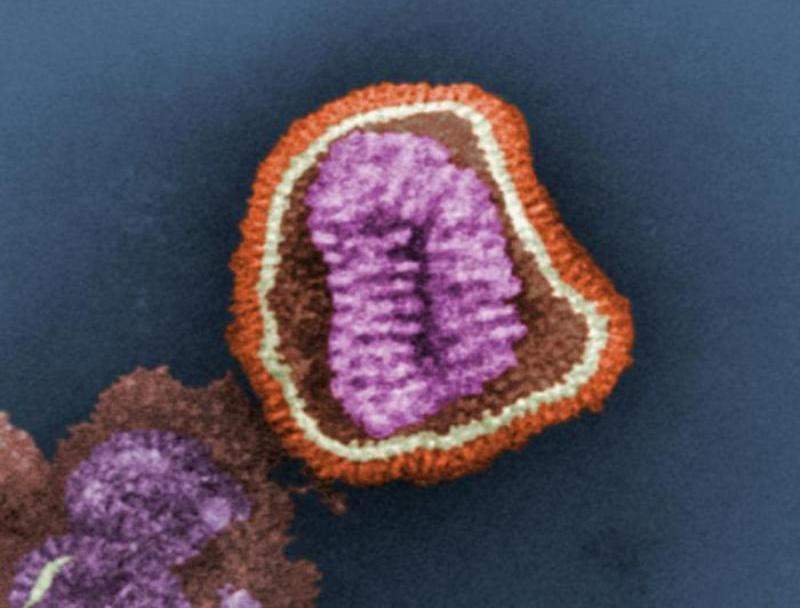
Novavax has begun a Phase ll clinical trial to evaluate the safety and tolerability of NanoFlu in comparison with two unnamed US-licensed comparators for the treatment of seasonal influenza.
The randomised, observer-blinded, active-controlled trial aims to study different doses of NanoFlu, both adjuvanted with the company’s Matrix-M adjuvant and unadjuvanted, against the comparators.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It also intends to select a dose/formulation of NanoFlu for a future pivotal Phase lll immunogenicity clinical trial.
As part of the Phase ll trial, around 1,375 healthy older adults are expected to be recruited across clinical sites in the US.
The trial has already enrolled its first subjects and is scheduled to provide top-line immunogenicity and safety data by the first quarter of next year.
Novavax president and CEO Stanley Erck said: “With top-line results expected in the first quarter of 2019, we plan to discuss these data with the FDA at an ‘End of Phase ll’ meeting and to agree on the appropriate Phase lll clinical trial design to support licensure via accelerated approval.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“We continue to believe NanoFlu is a differentiated flu vaccine capable of better addressing a global public health problem, and can thereby capture a significant share of the multi-billion-dollar seasonal influenza vaccine market.”
NanoFlu is a recombinant haemagglutinin (HA) protein nanoparticle influenza vaccine developed by Novavax using its saponin-based Matrix-M adjuvant.
According to a report published in February this year, NanoFlu demonstrated positive top-line results from a Phase l/ll clinical trial NanoFlu against Fluzone HD in older adults.
NanoFlu showed an acceptable safety profile and short-term reactogenicity compared with Fluzone HD.





