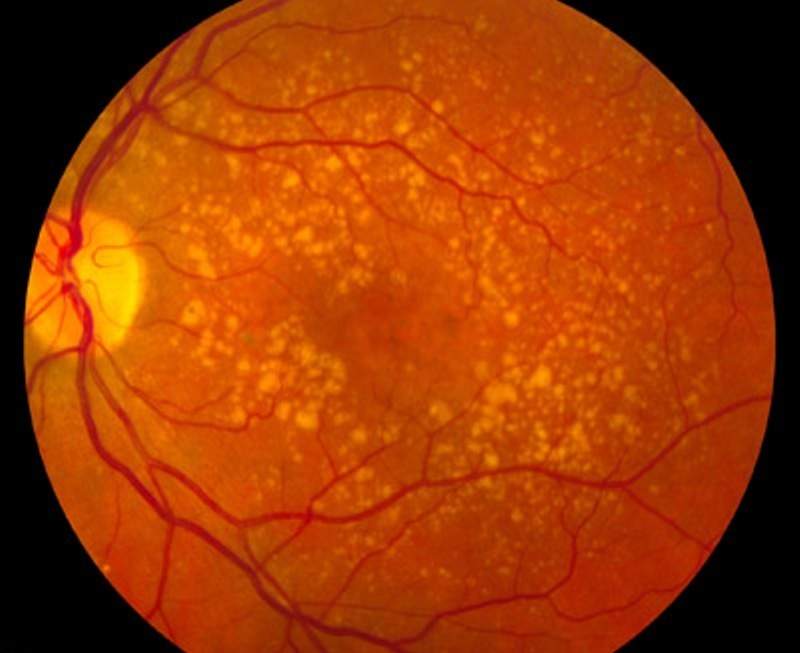
Ophthotech has completed patient enrolment in its Phase llb clinical trial of Zimura (avacincaptad pegol) to treat patients with geographic atrophy secondary to dry age-related macular degeneration (AMD).
A total of 286 patients have been enrolled for the randomised, double-masked, sham controlled multi-centre clinical trial.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The trial aims to evaluate the safety and efficacy of various doses of Zimura over a period of 12 months, followed by treatment and monitoring of patients for 18 months.
Ophthotech chief medical officer Kourous Rezaei said: “Recent clinical data together with the pre-clinical scientific evidence implicating complement in various retinal diseases further invigorates our enthusiasm for the therapeutic potential of Zimura.
“We look forward to initial top-line data from this clinical trial, which is expected to be available during the fourth quarter of 2019.”
Zimura is developed to target and inhibit the complement protein C5.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe solution is also being studied in combination with anti-vascular endothelial growth factor (anti-VEGF) agent Lucentis (ranibizumab) at 0.5mg in treatment-naïve wet AMD patients as part of an open-label Phase lla trial.
The randomised, dose-ranging, open-label, uncontrolled, multi-centre trial has included 64 patients.
The trial is expected to provide initial top-line results by the end of this year.
Based on the results, Ophthotech will decide whether to proceed to a randomised, sham-controlled clinical study of Zimura combination therapy with anti-VEGF in wet AMD.
In addition, Zimura is being investigated in a Phase llb clinical trial in patients with autosomal recessive Stargardt disease (STGD1).



