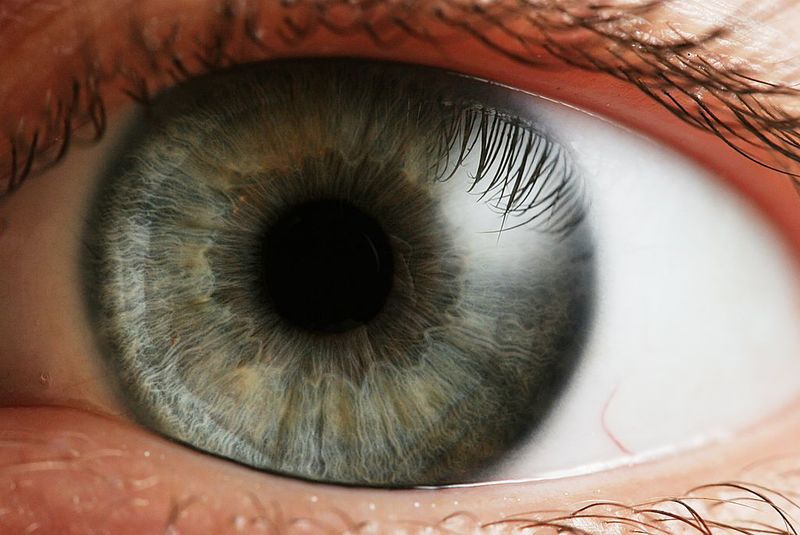
Australian biopharmaceutical firm Opthea has reported positive data from the Phase IIb clinical trial of OPT-302 in combination with ranibizumab (Lucentis) for the treatment of wet age-related macular degeneration (AMD).
The data has been presented at the European Society of Retina Specialists EURETINA 2019 Congress held in Paris.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It showed that the trial met its primary endpoint, showing superior vision gains with the combination treatment compared to sham plus ranibizumab, which was considered as control.
According to findings from additional analysis, the combination also led to a decrease in CST, sub-retinal fluid, intra-retinal cysts, total lesion area and choroidal neovascularisation (CNV) area.
Opthea CEO and managing director Dr Megan Baldwin said: “Together with the previously reported superiority in visual acuity gains, the further data analyses support the primary outcome of the study and demonstrate that OPT-302 has direct mechanistic effects on wet AMD lesion pathology.”
OPT-302 is a vascular endothelial growth factor receptor 3 (VEGFR-3) molecule designed to inhibit the activity of VEGF-C and VEGF-D proteins implicated in various retinal disorders.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe therapeutic is being developed in conjunction with VEGF-A inhibitors to facilitate the enhanced inhibition of disease pathways for better vision outcomes.
In combination with 0.5mg ranibizumab, 2mg and 0.5mg OPT-302 was assessed in 366 treatment-naïve patients who participated in the multi-centre, prospective, double-masked Phase IIb trial.
The therapy was given every four weeks for 24 weeks via intravitreal administration.
The safety profile of OPT-302 was observed to be similar to that of the control arm. The investigational combination was well tolerated without any safety concerns, Opthea noted.
Two ocular serious adverse events (SAEs) were reported in patients treated with 0.5mg OPT-302 plus ranibizumab. One adverse event was associated with trial discontinuation.
The company added that two patient deaths that occurred in the control group were not related to the study drugs.





