Panthera and Rutherford Health have formed a strategic collaboration to create a network of advanced clinical trial sites for developing new cancer treatments.
Under the partnership, known as Panthera@theRutherford, Panthera’s clinical trials managing expertise will be combined with Rutherford’s UK network of advanced centres and team of key oncologists.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Rutherford’s independent network currently comprises four cancer treatment centres. These centres are located in Newport, Reading, Bedlington and Liverpool.
Rutherford’s network will draw patients from all over the UK. It provides the Pharma and contract research organisations (CROs) access to patients through over 170 ‘referring oncologists’.
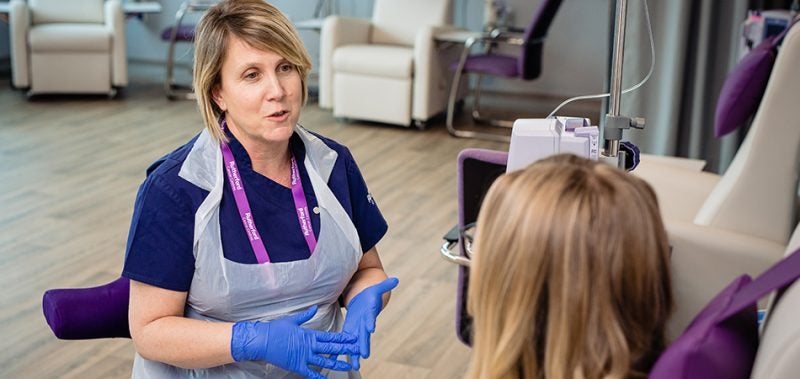
Each of the Rutherford Cancer Centres has state-of-the-art diagnostics including MRI, CT, and proton beam therapy, as well as offering chemotherapy, Immunotherapy and radiotherapy treatments.
These facilities, combined with leading oncologists, Panthera’s clinical trial professionals, and an experienced cancer care team, ensure that patients receive the highest possible standard of care.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataRutherford Health PLC CEO Mike Moran said: “We are delighted to partner with Panthera to provide professional cancer research in our centres. By collaborating with Panthera and our team of oncologists, we will be at the forefront of research into innovative therapies and we’ll be able offer more opportunities to patients and access to life-saving treatments.”
Each Rutherford Cancer Centre has advanced diagnostics including MRI, CT, and proton beam therapy treatments. These centres also offer chemotherapy, immunotherapy, and radiotherapy treatments.
Panthera chief medical officer Ian Smith said: “This really is a breakthrough partnership.
“Over 30% of all global clinical trials planned for 2020 are cancer trials, bringing research and cancer care together in one place will provide pharma and CROs the opportunity to undertake clinical trials with unrivalled access to more than 40 types of cancer and have access to millions of potential patients when there is a great shortage of top-class sites.”





