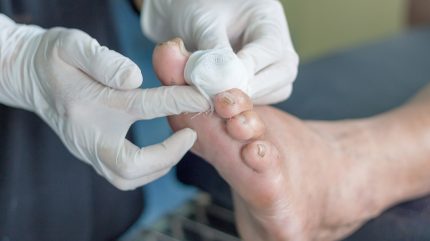
PolarityBio has completed the COVER DFUS II pivotal Phase III clinical trial of its autologous heterogeneous skin construct product, SkinTE, for treating Wagner 1 diabetic foot ulcers (DFUs).
The registrational, multi-centre randomised controlled trial (RCT) trial enrolled 120 participants at several US clinical sites over 14 months, averaging 8.6 patients per month.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
This enrolment rate surpassed previous DFU trial timelines. Subjects were randomised in a one-to-one ratio, administered either SkinTE plus standard of care (SOC) or SOC alone, followed over a 24-week period post-randomisation.
Eligibility criteria required patients to have a DFU for at least four weeks before screening and unresponsive to other treatments.
All subjects also underwent a two-week run-in period with SOC, with only those showing less than 30% change in wound size during this period qualifying for randomisation.
The primary efficacy endpoint of the trial is the rate of complete wound closure in 12 weeks.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSecondary endpoints focused on percent area reduction, time to healing, the proportion of participants experiencing treatment-emergent adverse events (TEAEs), total in-clinic or hospital days related to the index ulcer, and total days of controlled ankle motion (CAM) boot use.
PolarityBio chief clinical officer Ashlee Fishleigh said: “I want to personally thank our clinical research investigators and the dedicated research coordinators who have supported this programme.
“I also want to extend my sincere appreciation to the individuals who chose to participate in this trial. Your willingness to share your time, trust, and personal stories is what makes clinical research possible.”
The trial is designed to support a biologics license application (BLA) submission for SkinTE. Final results from the trial are expected in the first quarter of 2026.
Earlier in 2025, SkinTE received the US Food and Drug Administration (FDA) breakthrough therapy designation for Wagner Grade 1 DFUs.





