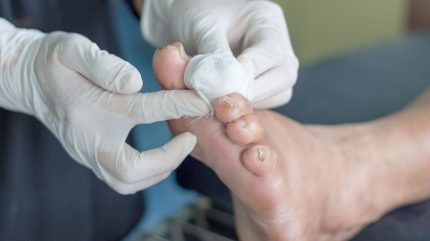
Regenerative medicine company RION has dosed the first subject in the Phase IIa clinical trial of its purified exosome product (PEP) for the treatment of diabetic foot ulcers (DFUs).
The multi-centre, prospective, open-label and randomised trial is set to enrol 40 subjects.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It aims to assess the safety and efficacy of PEP when applied topically to DFUs.
Participants will be divided into a 1:1 ratio to receive two vials of PEP, in addition to standard care (SoC), and the other half will receive SoC alone.
Evaluating the comparative effectiveness of PEP treatment for up to 12 weekly applications versus SoC is the trial’s objective.
PEP, which operates through a polyvalent mechanism, is crucial for tissue repair in DFU patients.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe product is a lyophilised powder made from human platelets and contains stabilised platelet-derived regenerative exosomes.
It is designed to encourage cell proliferation and new blood vessel formation, reduce inflammation, and offer cellular protection.
A successful outcome from the Phase IIa trial could lead to a pivotal study and eventually a biologics licence application for the product to treat DFUs.
RION co-founder Dr Atta Behfar said: “Dosing the first subject in the Phase II trial represents a key clinical advancement of our lead programme and is an important moment for both the company and the field of exosome science.
“With the Phase II effort, Rion has established key advances in exosome manufacturing and scaling, while cementing its commitment to revolutionising the treatment of chronic wounds. This pioneering exosome therapy is a testament to the potential of PEP to radically improve advanced wound care paradigms and patient outcomes.”



