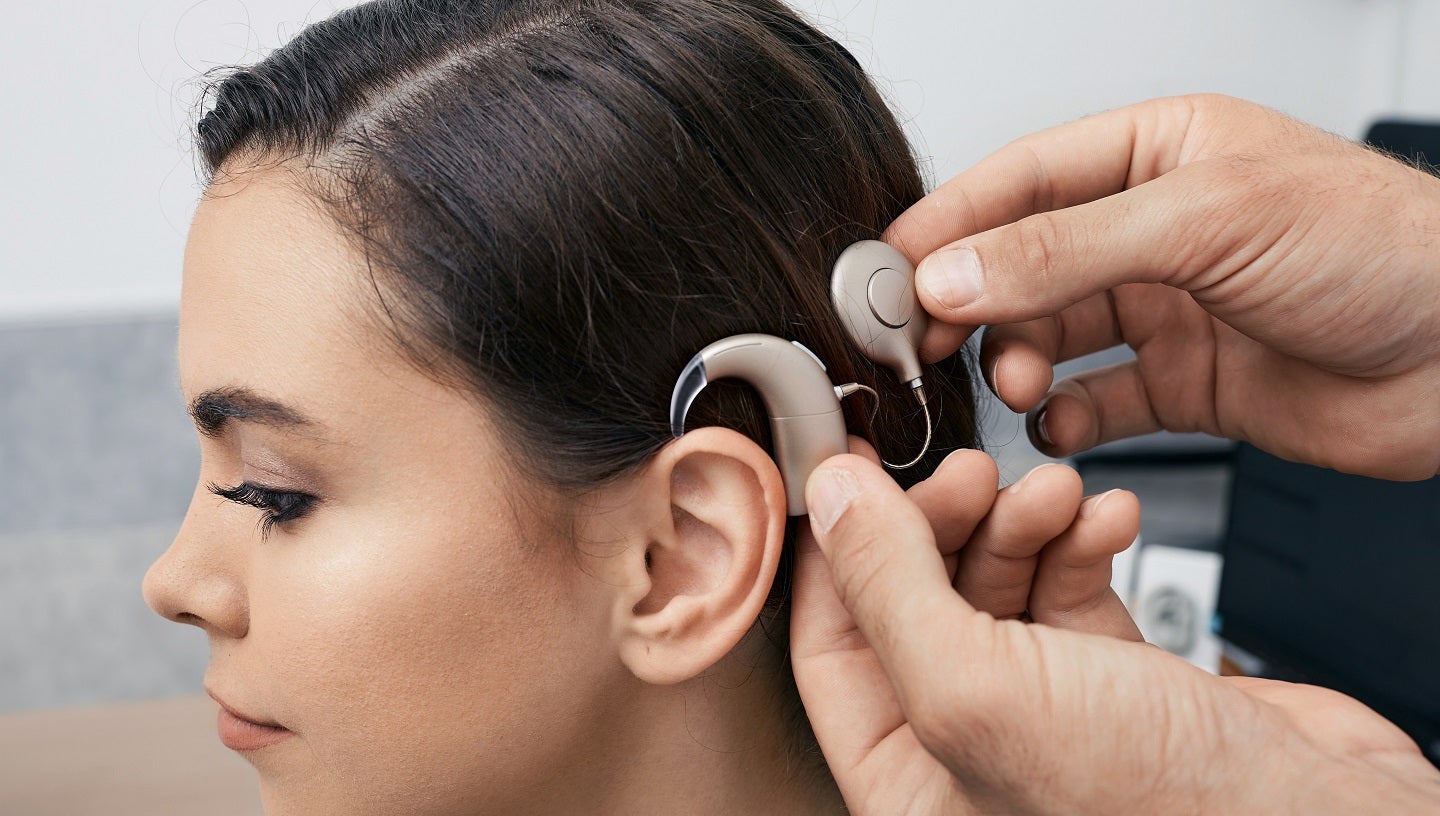
Sensorion has unveiled promising preliminary results from its Proof of Concept (POC) Phase IIa trial of SENS-401 (Arazasetron) for the prevention of residual hearing loss after cochlear implantation.
The multicentric, controlled, randomised, open-label study intends to evaluate the presence of SENS-401 in the cochlea (perilymph) after seven days of oral administration two times a day in adult participants due to moderately severe to profound hearing impairment.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Five adult participants received treatment with SENS-401 seven days before implantation and will continue to receive for further 42 days.
Evaluating change of hearing threshold from baseline to the end of the clinical trial in the implanted ear at several frequencies is the secondary endpoint of the study. The study will also assess several other secondary endpoints.
Seven days after the start of the study, all the patients showed presence of SENS-401 in perilymph at a level compatible with therapeutic efficacy.
Sensorion chief medical officer Géraldine Honnet said: “Presence of SENS-401 in the cochlea in 100% of patients having undergone cochlear implantation surgery confirms our confidence in the potential of our small molecule.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“These preliminary results support Sensorion’s ambition to provide solutions for people with hearing loss disorders.
“We will report further data from our Phase IIa study of SENS-401 in association with cochlear implants during our KOL webinar to be held on July 5, 2023.”
The Phase IIa study of SENS-401 is developed in collaboration with Sensorion’s partner Cochlear.
Sensorion is also planning to assess SENS-401 in a Phase II clinical trial for the prevention of Cisplatin-Induced Ototoxicity.





