A Boston Children’s Hospital trial in the US has found that adding chemotherapy to a treatment regimen such as catheterisation and surgery can detect abnormal cell growth and eventually help adolescents with pulmonary vein stenosis (PVS).
The trial initially included ten patients as a pilot and then expanded to enrol a total of 48 patients over a five-year period.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It was run by the Boston Children’s Hospital Pulmonary Vein Stenosis Programme with funding from Ansley’s Heart Endowment Fund, Christina Capozzi Memorial Foundation and TJ Reynolds Endowment Fund.
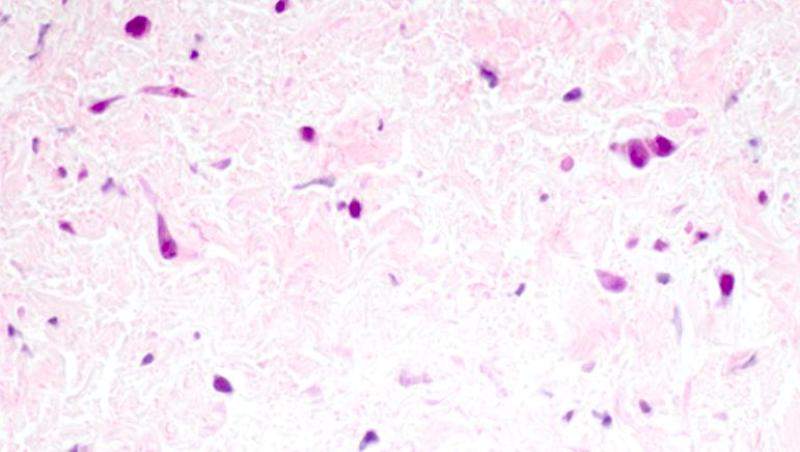
PVS is a rare disease where abnormal cells grow inside the veins responsible for carrying oxygen-rich blood from the lungs to the heart.
The disease usually affects young children by restricting blood flow through these vessels, and can completely seal them off if not treated.
The most severe form of PVS progresses very quickly and can lead to death within a matter of months after diagnosis.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataBoston Children’s Hospital representative Christina Ireland said: “Through this approach, we’ve created the first-ever population of survivors who are living with severe PVS.
“We’ve changed this disease from an acute killer to a chronic, manageable condition.”
Boston Children’s cardiologist and researcher Kathy Jenkins was senior author on the paper that outlines the trial results. She said that the sooner patients were able to start chemotherapy, the better their chances of recovery.
Jenkins said: “By the 48th week of treatment, we were able to stabilise PVS progression and prevent further lung damage in 31% of the children we treated.
“At 72 weeks, 77% of patients treated per our protocol were still alive.”
Dana-Farber/Boston Children Cancer and Blood Disorders Center neuro-oncologist Mark Kieran and a team of collaborators, including Boston Children’s pathologist Sara Vargas, were also involved in the trial.





