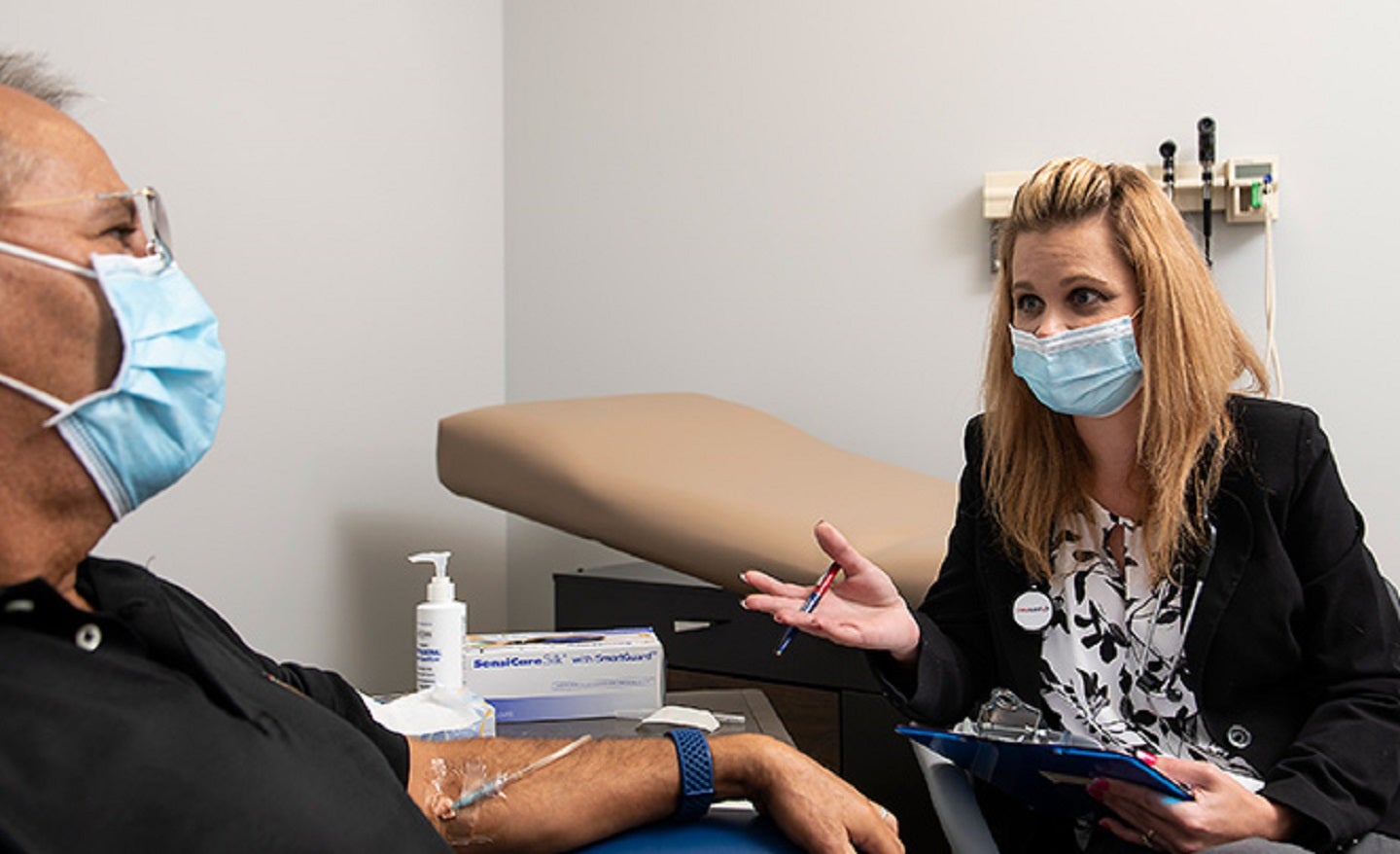
Researchers at the Center for Clinical Research (CCR) of the University of Kansas School of Medicine-Wichita in the US are employing various new ways to include additional rural people in Kansas in their clinical trials.
They are using in-person, telehealth, telephone and the US Postal Service to reach out to those people.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The rural residents can also take part in some clinical trials through video platform without travelling to Wichita.
Situated within KU Medical Center’s Wichita campus, CCR is part of the KU Medical Center Clinical and Translational Science Units that offers services to researchers.
CCR accommodates a laboratory, pharmacy and two exam rooms along with researchers and study coordinators.
Scientists at the research facility are handling multiple medical challenges, including Alzheimer’s disease research, treatment-resistant depression as well as prevention and treatment of Covid-19.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataUniversity of Kansas School of Medicine-Wichita internal medicine associate professor and CCR director Tiffany Schwasinger-Schmidt said: “Clinical trials open up potential opportunities.
“In a lot of the clinical trials that we do, people have tried other treatments and they haven’t been successful, or there just haven’t been a lot of treatments available.
“Just having the opportunity to try something — that’s a significant opportunity for patients.”
Schwasinger-Schmidt further noted that rural people’s environmental factors are different from those living in urban or suburban areas. These factors should be evaluated in clinical trials.
She added: “I think there’s a misconception that people who move to rural areas are looking for more solitude.
“And while there is some peacefulness and solitude out in the country, that doesn’t mean you shouldn’t have access to cutting-edge therapies.”





