Individual nationals and collective member states are working hard to make the European Union (EU) a more attractive area for clinical trials.
The region has seen a drop in recent years in the number of studies being conducted in the region, which Lene Grejs Petersen, senior adviser to EU CT-policy at the Danish Medicines Agency (DMA), attributed to several issues within the multi-regulatory framework.
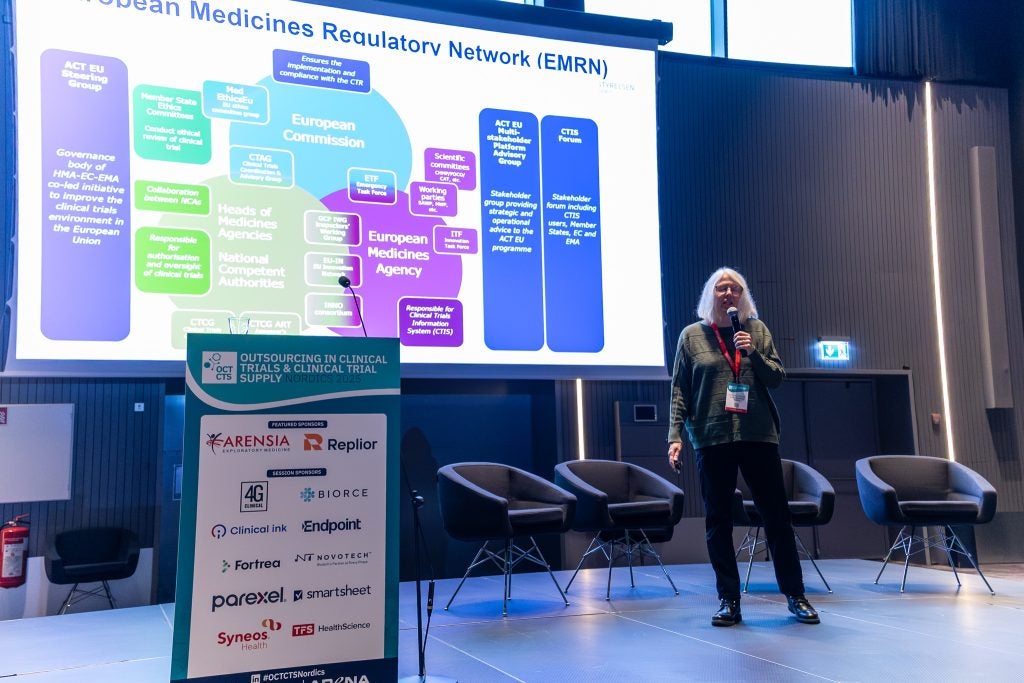
Speaking during the opening keynote session at Arena International’s Outsourcing in Clinical Trials and Clinical Trials Supply Nordics, in Copenhagen, Denmark, on 21 and 22 October, Petersen said that long timelines are likely a significant reason that sponsors are avoiding the region.
“The EU has very long timelines of up to 120 days,” Petersen said.
“Then, when you have already had the process long processes with the first application, if you need to resubmit, the process takes nearly as long as it did initially – for a sponsor, this is not very attractive, that's for sure.”
Petersen raised awareness to a report by the European Medicines Agency (EMA), Heads of Medicines Agencies (HMA), and the European Commission (EC), titled ‘Monitoring the European clinical trials environment’ which provides updated data from the Clinical Trials environment in the EU.
GlobalData analysis shows that while the US remains the dominant region for clinical trials, there has recently been an uptick in Asia, especially China.
GlobalData is the parent company of Clinical Trials Arena.
Other issues raised by Petersen include misalignment between parts one and two of the reviews, inconsistency within submission requirements across member states and the complexity of the review process and Clinical Trials Information System (CTIS) limitations.
There is hope on the horizon, however, as member states are coming together to try and rectify some of these limitations, Petersen confirmed.
This includes clarifying D17 requirements, such as ensuring what is mandatory and non-mandatory protocol content. Petersen also noted how the EU is making considerations for deletion or retention, which included harmonising reporting member state considerations, and strengthening the role of the reporting member state by defining expectations, responsibilities and boundaries for the reporting member state under Clinical Trials Regulation (CTR).
The Biotech Act is also open for comment from parties in clinical trials, which seeks to simplify regulations and expedite the process of bringing new products from the lab to the market.
Beyond the EU as a whole, Petersen pointed to the strengths of Denmark as a nation, which she said conducts the most clinical trials per million inhabitants within the EU.
Denmark’s notoriety in the clinical trial sector is heavily supported by its parliament, which has a strong focus on the sector, as well as Trial National, a single portal to apply for clinical trials in Denmark, to make it easier for sponsors.
Petersen added: “We have a strong Life Science strategy, where we have a focus on new strategies.
“We are quite pragmatic, and we have a new process where we can have 14 days of assessment for Phase I and integrated Phase II trials for multinational trials.”