Finnish biopharmaceutical Faron Pharmaceuticals has posted updated results of its phase I/II clinical trial for blood cancer drug bexmarilimab.
The drug is a novel immunotherapy aimed at patients with relapsed/refractory (r/r) acute myeloid leukaemia (AML) and myelodysplastic syndrome (MDS) after having failed Hypomethylating agents (HMA). It targets the Clever-1 immunosuppressive receptor found on macrophages, making them visible to the immune system.
Previous results for bexmarilimab have been incredibly positive, and the new posting suggests this was no fluke. Of the 22 patients in the cohort, 50% entered remission in doublet dose, and the drug remains well-tolerated at all tested dose levels.
The best results were in the prior HMA-failure MDS group, in which four out of five patients entered remission.
While overall remission rates are not yet known for the drug, Faron reports that the longest remission it has seen after treatment is 16+ months.
Company CEO Dr Markku Jalkanen highlighted the importance of this study, noting: “[R/r] AML and MDS … are conditions with dire prognosis and limited new therapies in the last decades.”
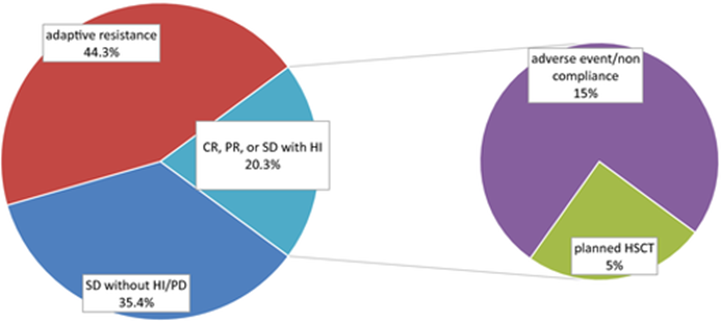
With current treatments, only 5% of patients with higher-risk MDS end up eligible for hematopoietic stem cell transplantation (HSCT), which intends to reset a patient's immune system, increasing survival outcomes. The five-year survival rates for AML and MDS between 2012 and 2018 were 30.5% and 36.9%, respectively.
Explaining the development process of bexmarilimab, Faron COO Dr Juho Jalkanen told Clinical Trials Arena: “Bringing the drug into the clinic, we started out with a rather traditional first-in-human approach in advanced solid tumours and did see encouraging activity in a number of indications there too, but then we got this AML and MDS trial going and started seeing extremely exciting results.
“For now, we are pursuing blood cancers because of the huge unmet need in r/r AML and MDS, and one of our biggest supporters being the Leukemia & Lymphoma Society.”
There are also plans to continue research into the drug’s efficacy on solid tumours, with Dr Jalkanen suggesting it could be a “game changer” in this field too. For now, however, the company is continuing to focus on blood cancers: “As a small biotech we cannot be pursuing everything at once.”
As Faron has yet to bring a drug to market, it remains dependent on private investment, and this news is likely to bolster confidence in its long-term prospects.
Our signals coverage is powered by GlobalData’s Thematic Engine, which tags millions of data items across six alternative datasets — patents, jobs, deals, company filings, social media mentions and news — to themes, sectors and companies. These signals enhance our predictive capabilities, helping us to identify the most disruptive threats across each of the sectors we cover and the companies best placed to succeed.









