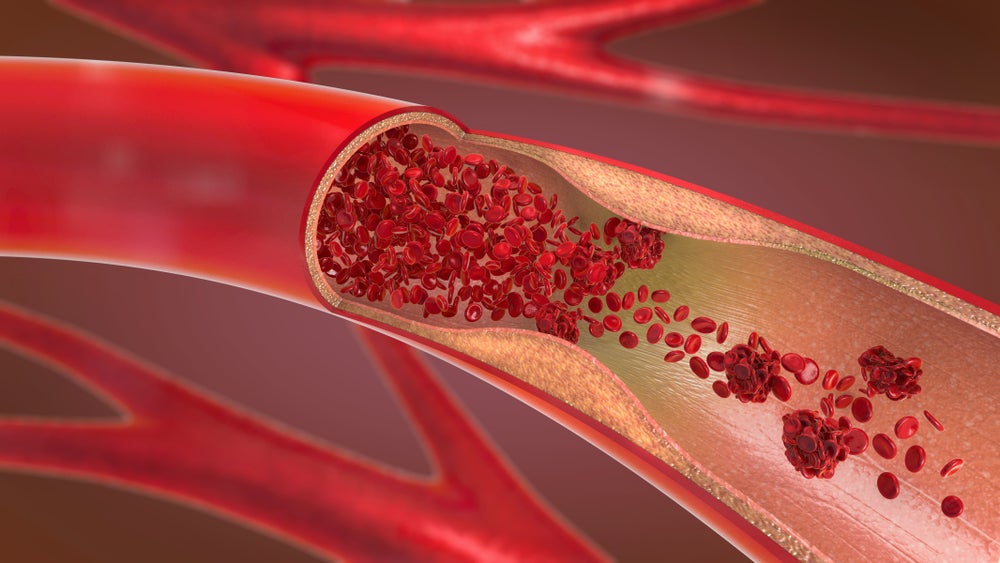
US-based Teleflex has completed enrolment in a trial investigating the safety and effectiveness of its Ringer perfusion balloon catheter therapy for patients undergoing percutaneous coronary intervention (PCI).
The prospective, multicentre, single-arm study (NCT04862689) has enrolled a total of 60 adult patients undergoing non-emergent PCI - a non-surgical procedure used to open clogged coronary arteries. The study will assess the use of the device in coronary intervention patients who might, according to the physician’s estimation, benefit from continued perfusion during coronary balloon inflations.
The primary endpoint of the study is the effectiveness of device success in dilatation/pre-dilatation of coronary stenosis during PCI. Patients will also be assessed for complications related to the device during the procedure and for any major cardiac adverse events.
Teleflex’s medical director Dr Christopher Buller said: “Completing enrolment is a significant milestone in the clinical validation and regulatory pathway to release the Ringer perfusion balloon catheter to treat patients.”
Also running in parallel is another study (NCT04849169) investigating the device in managing bleeding complications during a PCI procedure. Patients enrolled across sites in the US and Canada are those undergoing a standard PCI and who have a coronary perforation in their vessel during the procedure.
A perforation in the vessel is an unlikely, but potentially life-threatening, complication of a PCI procedure. The Ringer perfusion balloon catheter will be used in the event of a bleed if the physician deems it appropriate. The device is intended to control bleeding until permanent treatment is required, with the balloon able to be inflated for one hour.
Teleflex recently experienced issues with a catheter device made under its subsidiary Arrow International. The company issued a recall for more than 260,000 devices in June 2023.