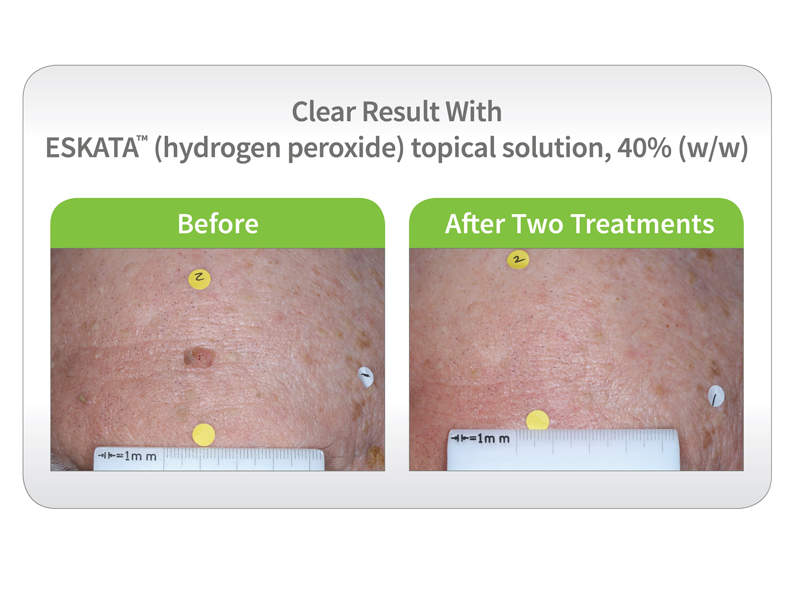
Eskata® (hydrogen peroxide) is a topical solution developed by Aclaris Therapeutics, a US-based pharmaceutical company, to treat raised seborrheic keratoses (SK) in adult patients.
It is a high-concentration hydrogen peroxide formulation designed for in-office treatment by healthcare professionals. It is a targeted treatment applied directly to the raised SK using a pen-like applicator.
Eskata is available as a clear and colourless solution containing 40% hydrogen peroxide.
Regulatory approval for Eskata
Aclaris submitted a new drug application for Eskata in February 2017, which was accepted for review by the US Food and Drug Administration (FDA) in May and approved in December of the same year.
In April 2018, Aclaris collaborated with Cipher Pharmaceuticals, a Canadian pharmaceutical company, to commercialise A-101 40% topical solution in Canada for the treatment of elevated SK. A new application Cipher submitted for the drug was accepted for review by Health Canada in December 2018.
Eskata was launched commercially in the US in mid-2018. Aclaris partnered with McKesson, a US-based healthcare company, to support its sales team in distributing Eskata.
Aclaris secured marketing approval for the Eskata cutaneous solution, 685mg from the Swedish Medical Products Agency to treat SK that are not pedunculated and have a maximum diameter of up to 15mm each in adult patients in February 2019.
The drug is marketed in several European countries, with the brand name Eskata® in Finland, Iceland, Netherlands, Norway, Portugal, Spain, Sweden, Czech Republic and Belgium and with the brand name Eskeriele® in Austria, France, Germany, Ireland, Italy and the UK.
Seborrheic keratoses causes and symptoms
SKs are benign skin growths that are most frequent in middle-aged and elderly people. They can be flesh-coloured, pink, yellow, grey, tan, brown or black and can range in size from a millimetre to a few centimetres broad. They have a slightly raised, waxy, or scaly look.
SKs can occur everywhere on the body excluding the palms, soles and mucous membranes. They first appear as small rough bumps, but often thicken and grow into a wart-like lump.
SKs are estimated to affect approximately 83 million adults in the US and are more widespread than skin diseases such as acne, psoriasis and rosacea.
Eskata’s mechanism of action
Eskata contains a high-concentration hydrogen peroxide formulation as a 40% weight/weight topical solution. It is designed to penetrate the SK lesions and cause oxidative damage so that the cells can be shed.
Hydrogen peroxide’s biocidal actions are assumed to be a result of the Fenton reaction, which produces free hydroxyl radicals.
In vivo, free radicals ultimately cause oxidative damage to proteins and membrane lipids.
Clinical trials on Eskata
The FDA approval for Eskata was based on results obtained from two pivotal Phase III clinical trials named SEBK-301 and SEBK-302.
The clinical studies enrolled a total of 937 patients with four target SK lesions across 34 centres in the US.
Out of a total of 937 patients, 467 were administered with Eskata and the remaining 470 were administered placebo.
The primary endpoint of both the clinical studies was the percentage of patients treated with Eskata, who achieved clearance of all four target lesions.
The studies’ results demonstrated that patients treated with Eskata were either cleared or nearly cleared of all SKs after two treatments, compared with the patients who received a placebo.
In the SEBK-301 study, 4% of the patients administered with Eskata achieved clearance, compared to 7.8% of the patients treated with Eskata in the SEBK-302 study.
Patients administered with a placebo in both studies did not achieve clearance of all four target SK lesions.
The results also showed that 13.5% of patients treated with Eskata achieved clearance of at least three of the four target SK lesions in the first clinical trial, compared with 23% of patients in the second study.
The overall combined trial results showed that 51.3% of lesions treated with Eskata were either clear or near clear at the completion of the trial, compared to 7.3% of lesions in the placebo group.
Furthermore, the studies showed that 65.3% of lesions on the face treated with Eskata were evaluated as clear or near clear, compared with 10.5% of lesions in the placebo group.
Eskata was generally well tolerated in the clinical studies with the most common reactions being itching, stinging, crusting, swelling, redness and scaling at the site of application.