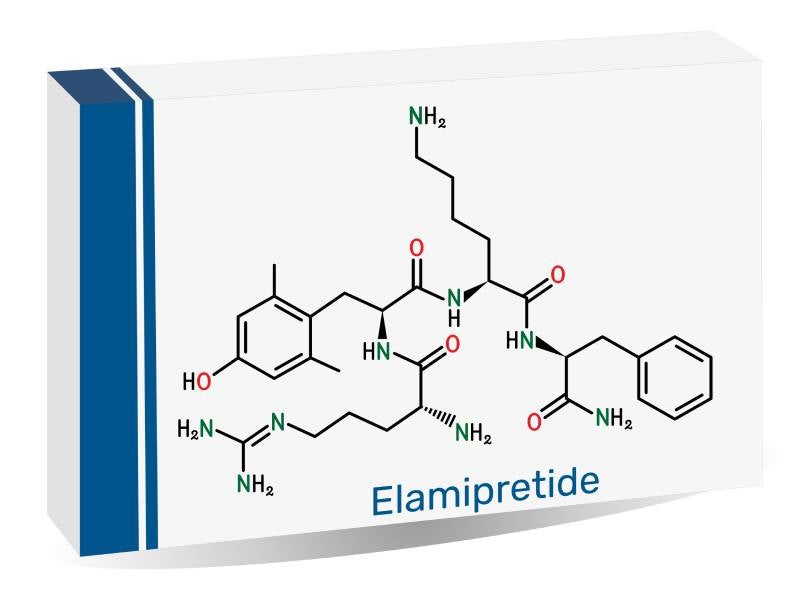
FORZINITY™ (elamipretide HCl) is indicated to enhance muscle strength in adult and paediatric patients with Barth syndrome who weigh at least 30kg.
Developed by Stealth BioTherapeutics, the drug is the first available treatment for Barth syndrome and the first mitochondria-targeted therapy to gain approval.
FORZINITY is supplied as a 280mg/3.5ml sterile, clear, colourless-to-yellow aqueous solution in single-use vials.
The drug is authorised for use in paediatric and adult patients who weigh 30kg or more. Stealth plans to collaborate with the US Food and Drug Administration (FDA) to gather further data in children under 30kg and to obtain qualification for its preservative-free formulation that has been used in expanded access for newborns.
Until a possible label extension is granted and the preservative-free formulation is formally qualified, Stealth will maintain compassionate-use availability for patients under 30kg who are already participating in its expanded access programme, as well as for those requiring emergency access.
Regulatory approvals for FORZINITY (elamipretide)
The FDA awarded elamipretide Fast Track Designation in 2017, Orphan Drug Designation in 2018 and Rare Paediatric Disease Designation in March 2020 for the treatment of Barth syndrome.
The European Medicines Agency granted Orphan Drug Designation for the drug in June 2021 for the treatment of Barth syndrome.
Stealth BioTherapeutics first submitted a New Drug Application (NDA) for elamipretide to the FDA in August 2021.
The NDA submission was supported by data from the SPIBA-001 Phase 3 Natural History Control Study, together with additional efficacy and safety evidence generated in the baseline‑controlled TAZPOWER Part 2 trial.
The FDA sent a refusal-to-file letter to Stealth BioTherapeutics as the NDA submission did not include any single adequate and well-controlled study capable of demonstrating efficacy.
Stealth BioTherapeutics resubmitted the NDA in January 2024, which was accepted for review by the FDA. The NDA was granted priority review status in May 2024.
In May 2025, the FDA issued a complete response, rejecting the NDA for the treatment of Barth syndrome and recommending resubmission of the NDA for accelerated approval based on improvements in knee extensor muscle strength as an intermediate clinical endpoint.
Stealth BioTherapeutics resubmitted the NDA in July 2025, and elamipretide was granted accelerated approval by the FDA in September 2025.
Barth syndrome causes and symptoms
Barth syndrome is an extremely rare inherited disorder caused by mutations in the TAFAZZIN gene, leading to cardiolipin abnormalities and mitochondrial dysfunction.
The disease results in exercise intolerance, muscle weakness, severe fatigue, heart failure, frequent infections and impaired growth.
It is associated with reduced life expectancy, with a high proportion of early deaths occurring by the age of five.
Barth syndrome primarily affects males and is estimated to occur in one in one million male births, with approximately 150 cases in the US.
Mechanism of action of FORZINITY
FORZINITY binds to mitochondrial cardiolipin and localises to the inner mitochondrial membrane, supporting mitochondrial structure and function.
It addresses cardiolipin deficiency and related electron transport chain issues, leading to improved adenosine triphosphate production, enhanced mitochondrial morphology and reduced formation of reactive oxygen species.
Clinical trials on FORZINITY
The FDA approval of FORZINITY was based on efficacy and safety data from the TAZPOWER clinical trial.
TAZPOWER was a Phase II, randomised, double-blind, placebo-controlled, crossover study comprising two parts.
Part 1 of the study was a randomised, double-blind, placebo-controlled trial that evaluated the safety, tolerability and efficacy of once-daily subcutaneous doses of 40mg elamipretide administered over a 12‑week period in individuals with Barth syndrome.
The two primary endpoints of the study were distance, in metres, achieved during the six-minute walk test (6MWT) and mean daily total fatigue score from the Barth Syndrome Symptom Assessment (BTHS-SA), calculated over the seven days immediately preceding study initiation.
Part 1 of the trial found no statistically significant difference between elamipretide and placebo on the primary endpoints.
Part 2 was a single-arm, open-label extension (OLE) study designed to investigate the long-term safety and tolerability, as well as longitudinal efficacy outcomes, of once-daily 40mg subcutaneous elamipretide given for up to 192 weeks.
Of 12 subjects enrolled for the TAZPOWER study, ten entered the OLE and eight completed the week-168 assessment. Participants in the OLE continued to receive elamipretide 40mg subcutaneously each day.
The primary endpoints of the trial were the long-term safety and tolerability of elamipretide up to 192 weeks.
Secondary endpoints included changes from baseline in the 6MWT and BTHS-SA total fatigue score, along with knee extensor muscle strength measured by handheld dynamometry, physician- and patient-reported outcomes, echocardiographic parameters, and biomarkers such as cardiolipin (CL) and monolysocardiolipin (MLCL).
Improvements in the 6MWT were observed at all OLE time points, with a cumulative increase of 96.1m at week 168 (P = .003). Mean BTHS-SA total fatigue scores remained below baseline at all OLE time points.
Three-dimensional left ventricular stroke, end-diastolic and end-systolic volumes showed improvement from baseline to week 168. MLCL/CL values also improved, corresponding with clinical outcomes.
The most frequently reported adverse reactions were injection site reactions, which may be managed with oral antihistamines or topical corticosteroids.
Other clinical trials on elamipretide
Stealth BioTherapeutics is also evaluating elamipretide for the treatment of ophthalmic conditions involving mitochondrial dysfunction, such as dry age-related macular degeneration, as well as for rare neuromuscular disorders including primary mitochondrial myopathy.