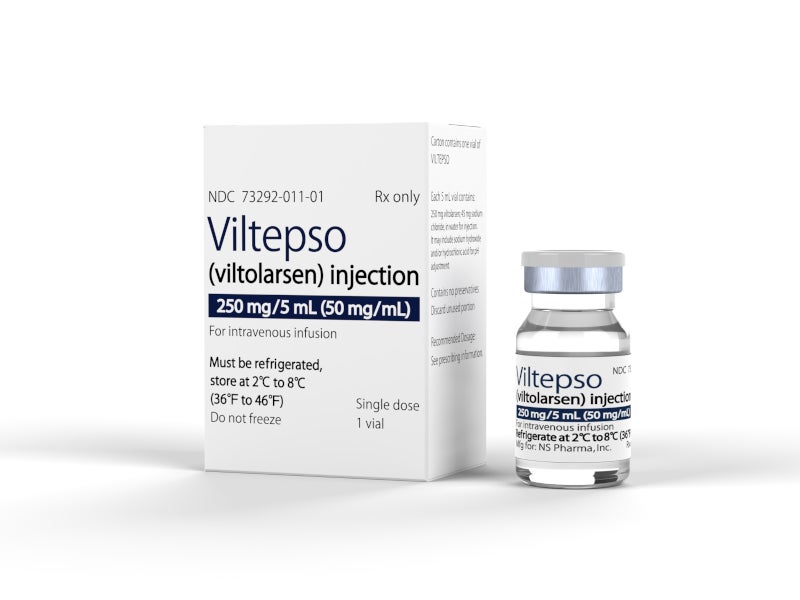
VILTEPSO™ (viltolarsen) is an antisense oligonucleotide drug indicated to treat Duchenne muscular dystrophy (DMD), a rare genetic disorder, in patients with a confirmed DMD gene mutation that is vulnerable to exon 53 skipping therapy.
Developed by NS Pharma, a wholly-owned subsidiary of Nippon Shinyaku, VILTEPSO (viltolarsen) is the first and only exon 53 skipping treatment to display elevated dystrophin levels in children as young as four years.
VILTEPSO is available in 250mg/5ml (50mg/ml) dosage strength in a single-dose vial as a clear and colourless solution for intravenous infusion over 60 minutes. The recommended dosage for administration is 80mg/kg once weekly.
VILTEPSO approvals
VILTEPSO received SAKIGAKE and Orphan Drug designations from the Ministry of Health, Labour and Welfare (MHLW) of Japan in 2015.
NS Pharma submitted a new drug application (NDA) for VILTEPSO to the MHLW in September 2019.
The drug also received Fast Track, Orphan Drug, and Rare Paediatric Disease designations from the FDA. VILTEPSO received marketing authorisation in Japan in March 2020 and was launched in May 2020.
The European Commission (EC) granted Orphan Drug designation to VILTEPSO for the treatment of DMD mutation in June 2020.
The US Food and Drug Administration (FDA) received an NDA for VILTEPSO in October 2019 and granted priority review status to the drug in February 2020. It granted accelerated approval to the drug in August 2020.
The approval was based on the improvement in dystrophin, the main protein for muscle health promotion in patients.
Duchenne muscular dystrophy (DMD) causes and symptoms
Duchenne muscular dystrophy is a type of severe muscular dystrophies, primarily occurring in males. It is a neuromuscular disorder that causes progressive skeletal, cardiac, and pulmonary muscular weakness and loss.
DMD is caused by genetic abnormalities, which inhibit the development of dystrophin. Early signs of DMD may include delayed sitting, standing, or walking capabilities. Multiple forms of genetic mutations can cause DMD.
The disease affects approximately one in 3,600 male infants globally.
DMD patients suffer from progressive and irreversible loss of muscle with signs beginning as early as the age of two. The condition leads to a progressive loss of mobility by adolescence and the patient may require a wheelchair.
Cardiac and respiratory issues begin in teenage years and progress to severe, life-threatening complications.
Viltolarsen mechanism of action
Viltolarsen is a morpholino antisense oligonucleotide drug that binds to exon 53 of dystrophin pre-mRNA and excludes the exon in patients with genetic mutations susceptible to exon 53 skipping during mRNA processing.
Exon 53 skipping is intended to allow the production of an internal dystrophin protein in patients with genetic mutations susceptible to exon 53.
Clinical trials on VILTEPSO
The FDA approval of VILTEPSO was based on two clinical studies enrolling a total of 32 patients. The improvement in dystrophin was evaluated in one of the two studies called Study 1.
Study 1 was a phase two, randomised, multi-centre, two-period, placebo-controlled clinical trial performed on 16 patients with confirmed DMD gene mutation that is amenable to exon 53 skipping.
The patients were initially randomised to receive either VILTEPSO or placebo for four weeks, followed by once-weekly administration of 40mg/kg VILTEPSO or 80mg/kg VILTEPSO to all patients for 20 weeks.
The primary endpoint of the trial was the change in dystrophin protein level from baseline at week 25.
Patients receiving 80mg/kg VILTEPSO showed an average increase in dystrophin levels from 0.6% of normal at baseline to 5.9% of normal by week 25. All the patients in a pivotal VILTEPSO trial showed an increase in dystrophin levels.
The most frequent side effects of VILTEPSO included cough, fever, upper respiratory tract inflammation, and injection site reactions.