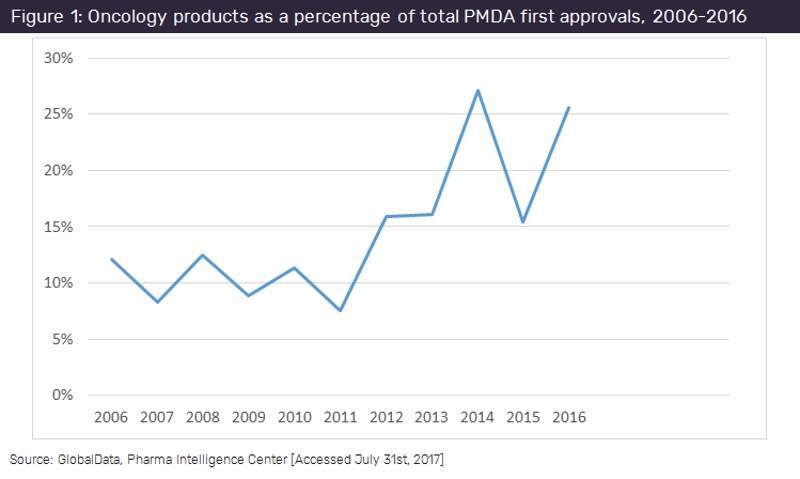

The number of oncology drugs approved by Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) as a percentage of total approvals has nearly doubled in the past ten years, reflecting the growing oncology focus in drug research and development (R&D) seen in recent years.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The oncology pipeline is deep in both early and late stage candidates, with developers keen to follow suit after the first wave of immuno-oncology approvals.
With the cancer market projected to see huge growth in the next decade, we are unlikely to see a reversal of this trend any time soon.





