No Filter Selected
No Filter Selected
No Filter Selected
No Filter Selected
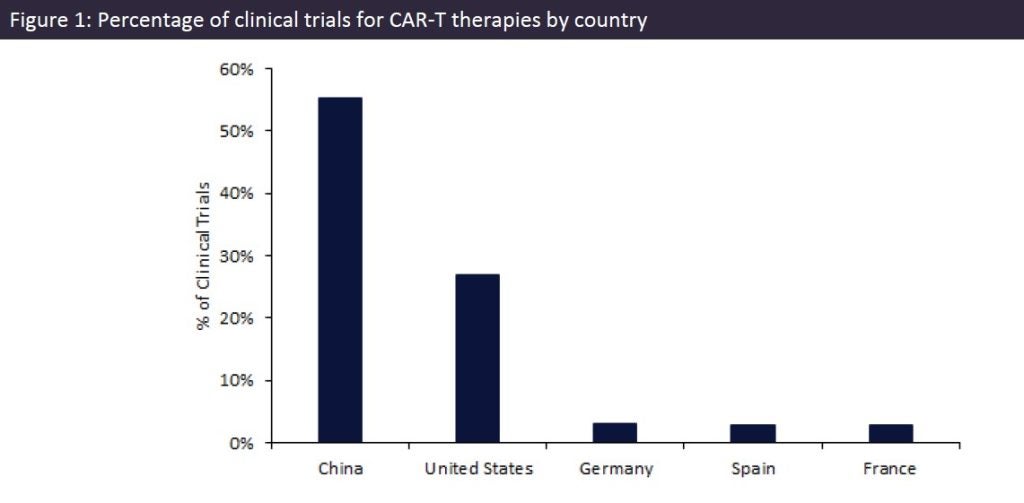
China dominates CAR-T therapies
The reason for the high number of trials in China could be due to government initiatives to ensure new treatments come to market.
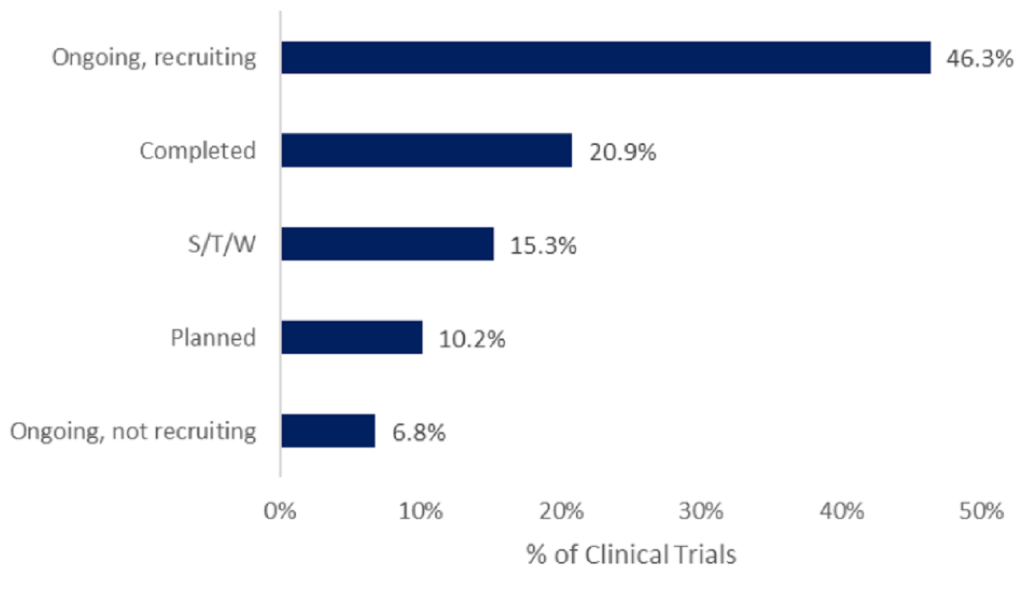
Most CAR-T trials are ongoing and recruiting participants
CAR-T therapies have shown promising results in certain types of blood cancer.
The increasing trend towards allogeneic cell therapy
As of January 17, 2022, GlobalData noted 542 active chimeric antigen receptor (CAR) T-cell (CAR-T cell) agents in the global...
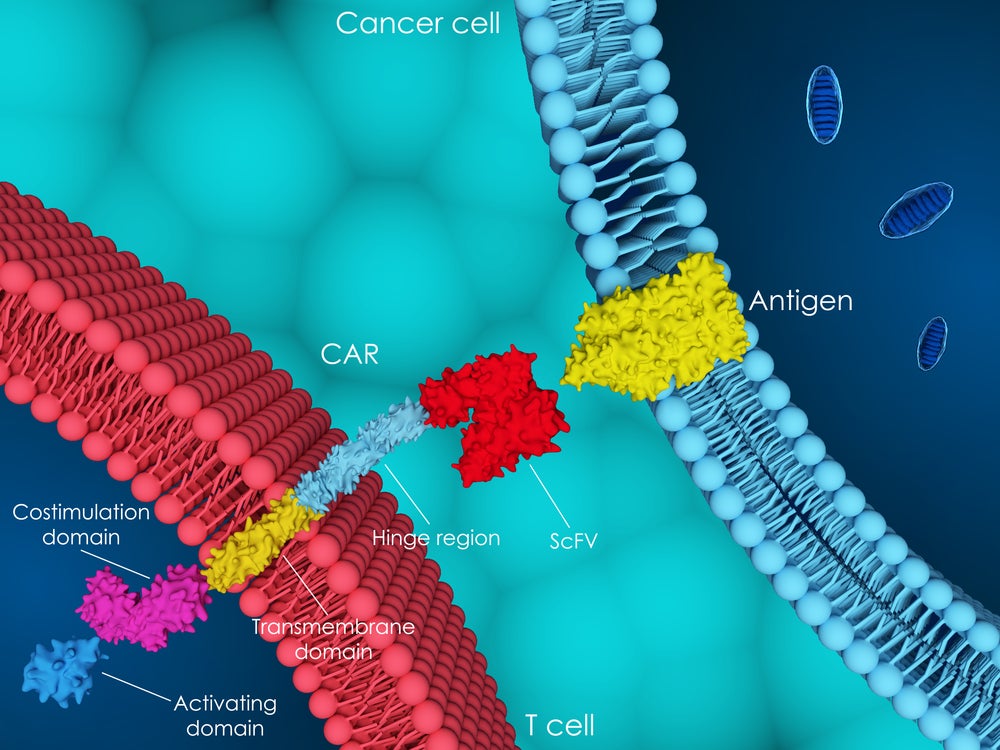
CAR-T landscape set to expand in 2024
CAR-T therapy is an expensive form of cancer treatment, with costs that can reach millions of dollars per patient.
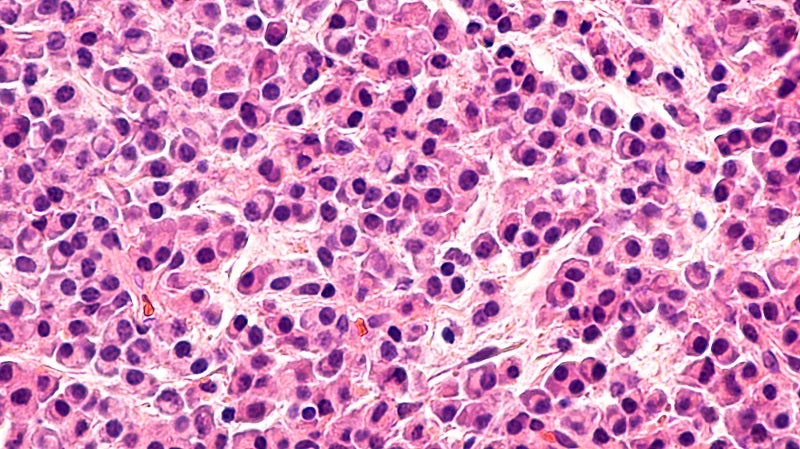
BMS positioned to lead in the multiple myeloma market
Chimeric antigen receptor T-cell (CAR-T) therapies have become standard of care for certain haematological malignancies due to their unprecedented specificity...
Mustang Bio and City of Hope start Phase I CAR T cells trial
Mustang Bio and cancer research and treatment centre City of Hope have started a Phase I clinical trial to investigate the safety and effectiveness of intraventricular delivery of CAR T cells to the brains of patients with HER2-positive breast cancer with brain metastases.
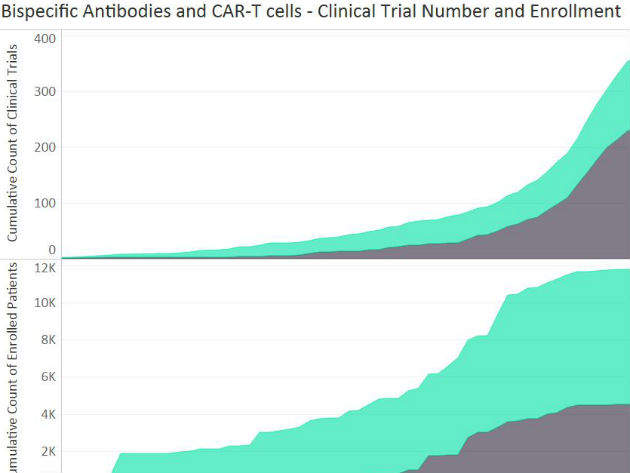
Bispecific antibodies and CAR-T cells: A comparison of historical trial numbers and patient recruitment
Since Novartis’ CTL019 received unanimous recommendation for approval in acute lymphoblastic leukemia (ALL) by the US Food and Drug Administration (FDA) advisory committee on the 12th of July 2017, the development of chimeric antigen receptors T cell (CAR-T) therapies has been watched carefully to anticipate the clinical and commercial impact of these immuno-oncology agents.
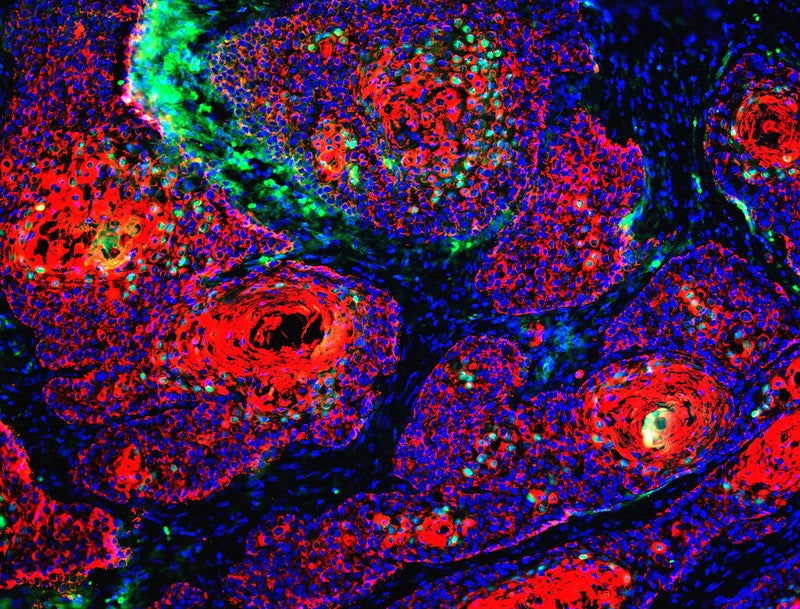
Actinium announces patient enrolment in CAR T-cell therapy trial
Actinium Pharmaceuticals has announced the initiation of patient enrolment in the Phase I study of Iomab-ACT for targeted conditioning before treatment with Memorial Sloan Kettering Cancer Center’s (MSK) CD19 targeted CAR T-cell 19-28z.
Innovation in immuno-oncology: Leading companies in cancer chimeric antigen receptor (CAR) T-cell therapy
The pharmaceutical industry continues to be a hotbed of innovation, with activity driven by the evolution of new treatment paradigms,...
PLAT-05 trial launches at Children's National Health System
Children's National Health System in the US has initiated the PLAT-05 clinical trial of a chimeric antigen receptor (CAR) T-cell immunotherapy to treat acute lymphoblastic leukaemia (ALL).
NeoImmuneTech presents data from Phase Ib trial of CAR-T therapy regimen
Adding NT-I7 offered a rise in the amplification of CAR-T cells, extended their presence in the body and enhanced T cell stemness.
Innovent doses first subject with solid tumour CAR-T asset
The trial will assess the safety, tolerability, pharmacokinetics and efficacy of IBI345 for Claudin18.2-positive solid tumour.
Complete responses reported for Nkarta’s off-the-shelf NK cell therapies
On April 25, Nkarta, a biopharmaceutical company developing off-the-shelf engineered natural killer (NK) cell therapies, announced highly promising results from...
ASCO 2023: Precigen reports positive early data for CAR-T therapy
Positive Phase I data could help propel development of Precigen’s CAR-T cell therapy PRGN-3005 in advanced ovarian cancer.

ImmPACT Bio begins dosing in Phase I/II lymphoma therapy trial
The open-label trial will assess the efficacy and safety of IMPT-314 in R/R aggressive B-cell lymphoma patients.

Nexcella reports new data for CAR-T therapy in AL amyloidosis
Nexcella’s CAR-T cell therapy program gains momentum with a small set of early positive data in AL amyloidosis.

HER2-directed CAR-T therapy is promising for glioblastoma but not breast cancer
A total of 32 HER2-directed CAR-T cell therapies are currently in the pipeline targeting HER2-positive tumors.
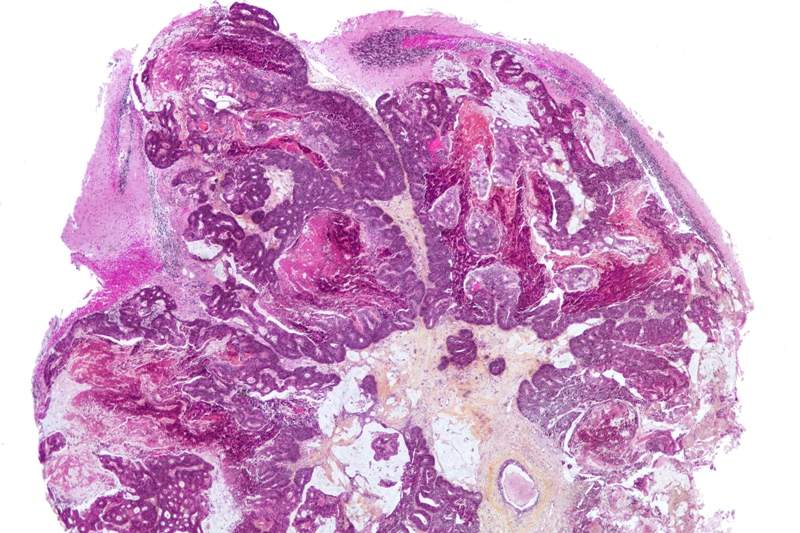
AACR 2023: Positive results of TRAVERSE – allogeneic CAR-T for R/R kidney cancer
Relapsed and refractory (R/R) renal cell carcinoma (RCC) presents a high unmet need.

Pfizer and Allogene to partner on allogeneic CAR T cell therapies
Pfizer and Allogene have announced a partnership to develop allogeneic chimeric antigen receptor T cell (CAR T) cell therapies, a method of cancer treatment based on investigational immune cell therapy.
Debating the Hype of CAR-T Therapy
S. Brandon Early, Greg Guarasci, Giovanna Chan, Cassandra Moran, MD, and Stephanie Gonzalez explore the strides made in CAR-T cancer treatments
