
Author: Manny Vazquez, Senior Director Clinical Data Strategy, Veeva
The number of data points collected in clinical trials has tripled over the last decade, with total amount of data captured nearly doubling. Adding to study complexity is the growing input from wearable devices, electronic clinical outcome assessments (eCOA), central labs, and other third-party data. The resulting surge in data volume is a double-edged sword for the people collecting and monitoring this data (and making sure it’s fit for purpose).
On one hand, a larger and more comprehensive dataset allows for more detailed analysis to better understand diseases and patient needs. But on the other, a greater volume of data places a significant burden on the patients providing it. Without the processes and systems to deal with the data, these richer insights also give way to friction and frustration for clinical data professionals, as legacy systems and workflows are designed for siloed, less variable data structures.
The cost is also high at the business level. For instance, industry research shows that both clinical data managers (CDMs) and clinical research associates (CRAs) spend a third more active time resolving a query on third-party data than a query on EDC data. Based on an average query costing between $28-225 to resolve and the average Phase III study generating 96,980 queries, inefficiencies in query management could be costing sponsors millions of dollars.
Clinical data managers and research associates bear the brunt of inefficiencies
CDMs and CRAs are disproportionately affected by workflow inefficiencies, which are systematically embedded in critical tasks such as data reconciliation and monitoring documentation. It is estimated that CDMs spend 12 hours per week per study on the two tasks ranked as the most inefficient by survey respondents: manual data reconciliation (52%) and data review and cleaning (35%). Three quarters of CDMs said too many manual steps are the primary cause of inefficiencies [Figure 1].
Nearly all CDMs confirmed that manual data reconciliation (97%) and data review and cleaning (91%) take place either outside of core clinical systems, or with a mix of systems. Performing critical functions outside of validated clinical systems — using generic tools like spreadsheets — adds to the manual burden already placed on clinical data professionals.
“We have home-grown tools for data cleaning, but I don’t trust them because they cannot handle the variability in data structure between studies. Data sources have differences in date formatting, extra spaces in an output, column naming and so on. They can differ by source and by study. There is too much variation, so we stick with Excel.” — Senior Clinical Data Specialist, Global CRO.
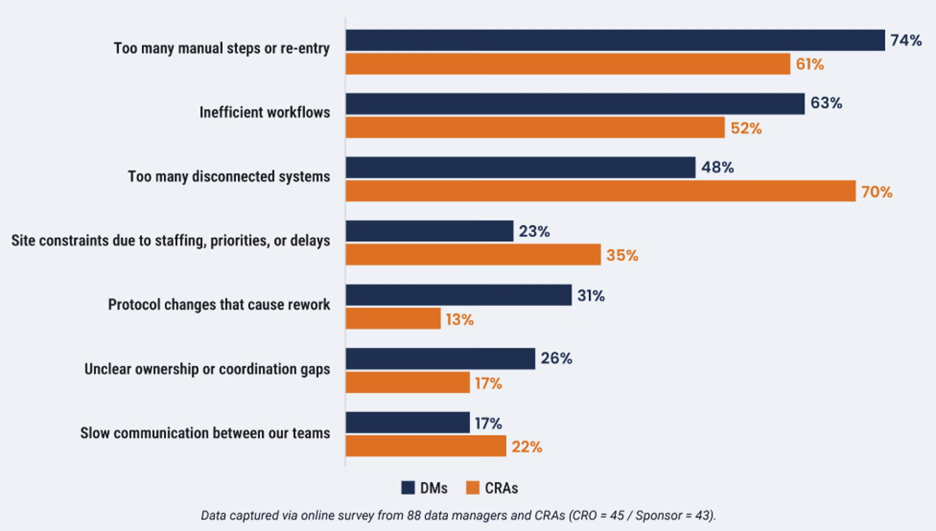
CRAs cite disconnected systems as their top driver of inefficiency (70%). This leads to a widespread reliance on manual trackers and time-consuming transcription between siloed platforms, such as documenting in non-validated systems (e.g., OneNote) before transcribing into a clinical trial management system (CTMS).
“It’s not that I like it, I don’t have any other tool to keep everything in one place. I have to capture [data] in a non-validated system.” — CRA Subject Matter Expert, Top 20 Biopharma Company
The cost of this inefficiency is stark: benchmark analysis shows that working in trackers such as Excel, OneNote, and Word account for the most time wasted over monitoring visit reports, which can amount to over $1m per 1,000 reports.
Consequences for people and the data
Inefficiencies don’t just make trials run slower and cost more money; there are risks to the data itself and the wellbeing of those working on it. Nearly all CRAs (91%) surveyed are concerned about burnout and staff turnover [Figure 2]. Unfortunately, this is not a new phenomenon: the turnover rate for CRAs increased from 24% in 2020 to 32% in 2022, with turnover intention linked to financial compensation and perceived workload.
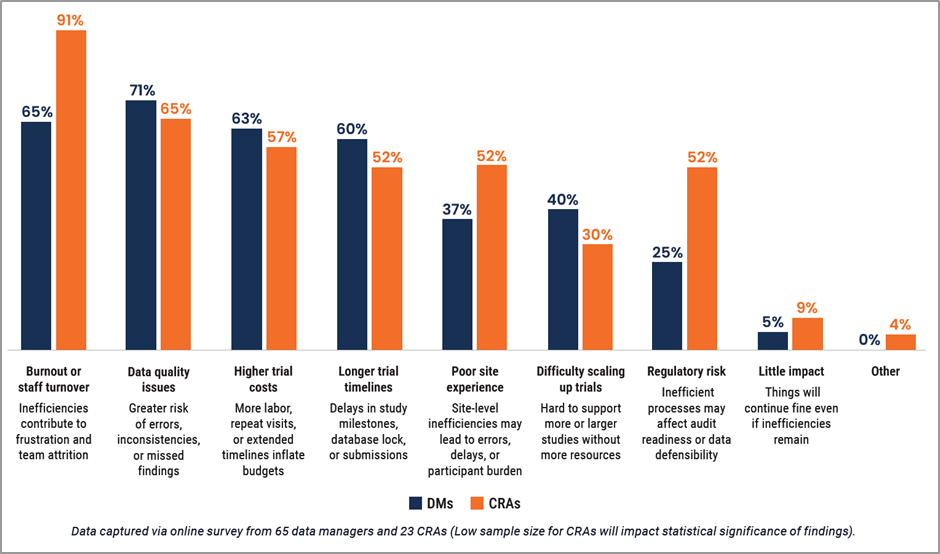
For CDMs, however, the concern over burnout and staff turnover (65%) is relatively new and together with the future threat to data quality (71%), creates implications for risk-based data management (RBDM) approaches. As RBDM becomes more mainstream, this potentially exacerbates data quality concerns as CDMs move away from reviewing 100% of the data. With burnout rising, it is unsustainable to continue reviewing all data to the same level.
A call-to-action for system integration and automation
Despite the challenges caused by inefficient processes and systems, almost half of data managers and CRAs say that resistance to change is a key barrier to a more productive future state. A sense of current workarounds being ‘good enough’ hides the damage caused by inefficiency in clinical trials and prevents a collective mindset shift to strive for better processes.
However, there’s optimism on the horizon. Three quarters of CDMs and over half of CRAs say their teams are actively looking for ways to modernize trial execution, and better integration between clinical data and operations systems is top of their list of improvements (81%). With a connected clinical infrastructure, data can freely flow between stakeholders, eliminating the need to reconcile data sources. This can provide CRAs with updates in real-time, and help study leaders to make better decisions based on complete and concurrent data.
The outcomes signals a clear call-to-action for clinical leaders: invest in tools that automate data review, reconciliation, and reporting to directly address the most frustrating manual tasks, and advance towards a culture of efficiency.
Read the full report for more data and insights.


